Abstract
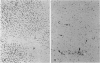
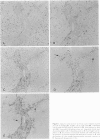
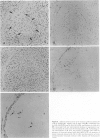
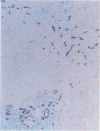
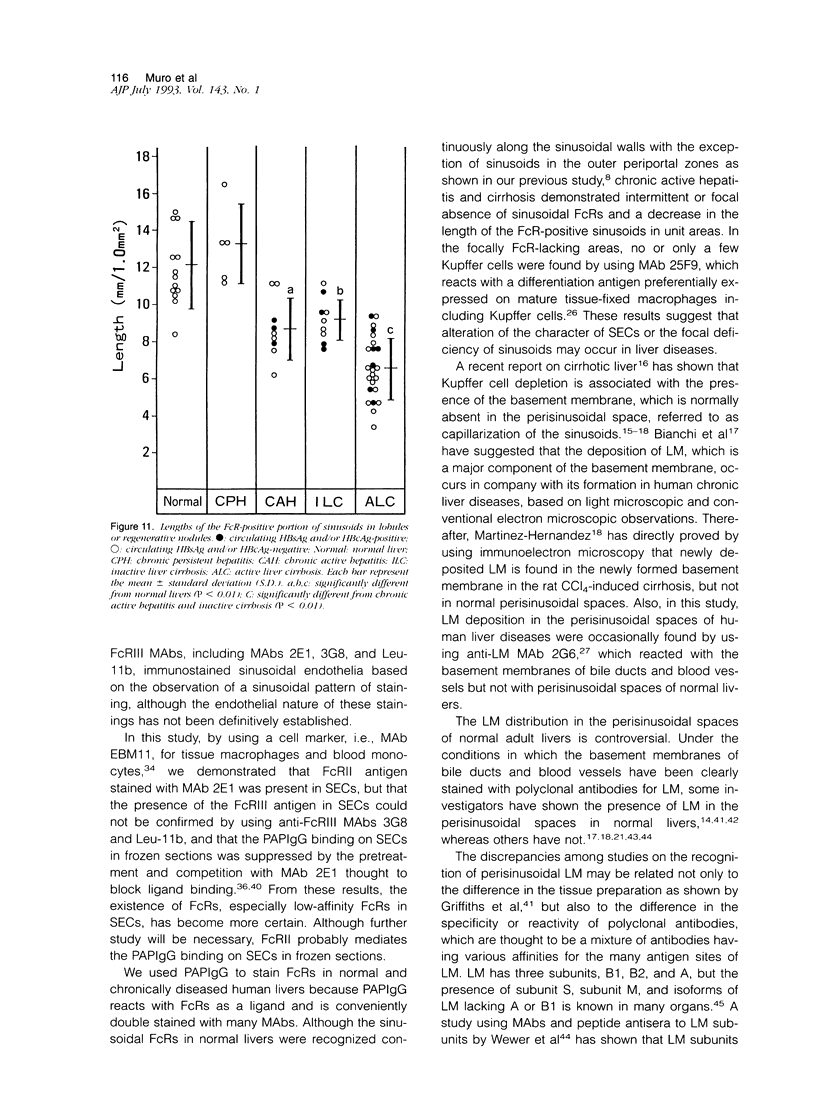
To analyze the pathological changes occurring in Fc receptors (FcRs) in sinusoidal endothelial cells (SECs) in chronic liver diseases, we first characterized immunohistochemically the SEC FcRs by using monoclonal antibodies (MAbs) to FcRs and then investigated the distribution of the SEC FcRs by using peroxidase-antiperoxidase IgG complexes as a ligand on frozen sections. MAb 2E1 to FcRII reacted with SECs in a similar manner to peroxidase-antiperoxidase IgG and blocked the peroxidase-antiperoxidase IgG binding to SECs, whereas MAbs 3G8 and Leu-11b to FcRIII did not. FcRs in normal liver were found along the sinusoidal walls, except for those in the outer periportal zones, but FcRs in chronic active hepatitis and cirrhosis were intermittently or focally absent. The lengths of the FcR-positive portion of sinusoids in unit areas were respectively about 54% and 76% of the normal values in active and inactive cirrhosis. Where FcRs were absent, the MAbs CD36, CD31, and EN4 revealed the presence of sinusoids and, in active cirrhosis, frequently the thickening of liver cell plates. The FcR-negative SECs in the outer periportal zones of normal livers were different from the SECs of other sites in the presence of PAL-E antigen and a rich amount of EN4 antigen, though these sinusoids possessed Kupffer cells and no perisinusoidal deposition of laminin. The FcR-negative SECs in liver diseases occasionally presented the character of ordinary blood vessels, viz., PAL-E antigen, CD34 antigen, and a deficiency of Kupffer cells, regardless of perisinusoidal laminin deposition. However, they preserved the character of normally FcR-possessing SECs, viz., CD36 antigen, and a small amount of EN4 and CD31 antigens. These findings indicate that the outer-periportal SECs in normal livers are phenotypically different from other SECs and that the SECs in diseased livers frequently undergo phenotypical changes, including loss of FcRs, regardless of perisinusoidal laminin deposition, i.e., capillarization of the sinusoids. These phenotypical changes in SECs may reduce the capacity of FcR-mediated IgG-IC metabolism in diseased livers.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Barnwell J., Silverstein R. L., Nachman R. L. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987 Apr;79(4):1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbs C., Haboubi N. Y., Mellor J. M., Smith A., Rowan B. P., Warnes T. W. Endothelial cell transformation in primary biliary cirrhosis: a morphological and biochemical study. Hepatology. 1990 May;11(5):723–729. doi: 10.1002/hep.1840110503. [DOI] [PubMed] [Google Scholar]
- Bianchi F. B., Biagini G., Ballardini G., Cenacchi G., Faccani A., Pisi E., Laschi R., Liotta L., Garbisa S. Basement membrane production by hepatocytes in chronic liver disease. Hepatology. 1984 Nov-Dec;4(6):1167–1172. doi: 10.1002/hep.1840040612. [DOI] [PubMed] [Google Scholar]
- Cui Y. C., Tai P. C., Gatter K. C., Mason D. Y., Spry C. J. A vascular endothelial cell antigen with restricted distribution in human foetal, adult and malignant tissues. Immunology. 1983 May;49(1):183–189. [PMC free article] [PubMed] [Google Scholar]
- De Leeuw A. M., Brouwer A., Knook D. L. Sinusoidal endothelial cells of the liver: fine structure and function in relation to age. J Electron Microsc Tech. 1990 Mar;14(3):218–236. doi: 10.1002/jemt.1060140304. [DOI] [PubMed] [Google Scholar]
- Engvall E., Davis G. E., Dickerson K., Ruoslahti E., Varon S., Manthorpe M. Mapping of domains in human laminin using monoclonal antibodies: localization of the neurite-promoting site. J Cell Biol. 1986 Dec;103(6 Pt 1):2457–2465. doi: 10.1083/jcb.103.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farace F., Mitjavila M. T., Betaieb A., Dokhelar M. C., Wiels J., Finale Y., Kieffer N., Breton-Gorius J., Vainchenker W., Tursz T. New hematopoietic differentiation antigens detected by anti-K562 monoclonal antibodies. Cancer Res. 1988 Oct 15;48(20):5759–5765. [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y., Nagura H., Imoto M., Koyama Y. Immunohistochemical studies on structural changes of the hepatic lobules in chronic liver diseases. Am J Gastroenterol. 1986 Dec;81(12):1149–1155. [PubMed] [Google Scholar]
- Griffiths M. R., Keir S., Burt A. D. Basement membrane proteins in the space of Disse: a reappraisal. J Clin Pathol. 1991 Aug;44(8):646–648. doi: 10.1136/jcp.44.8.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
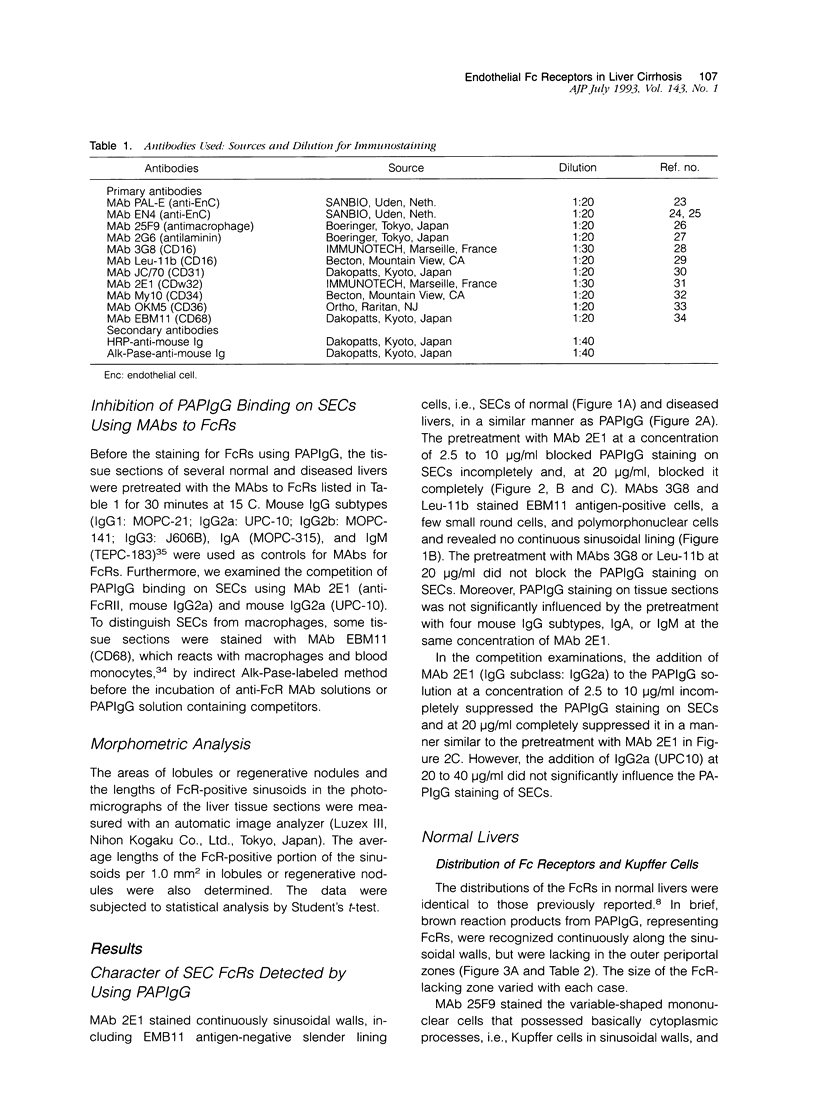
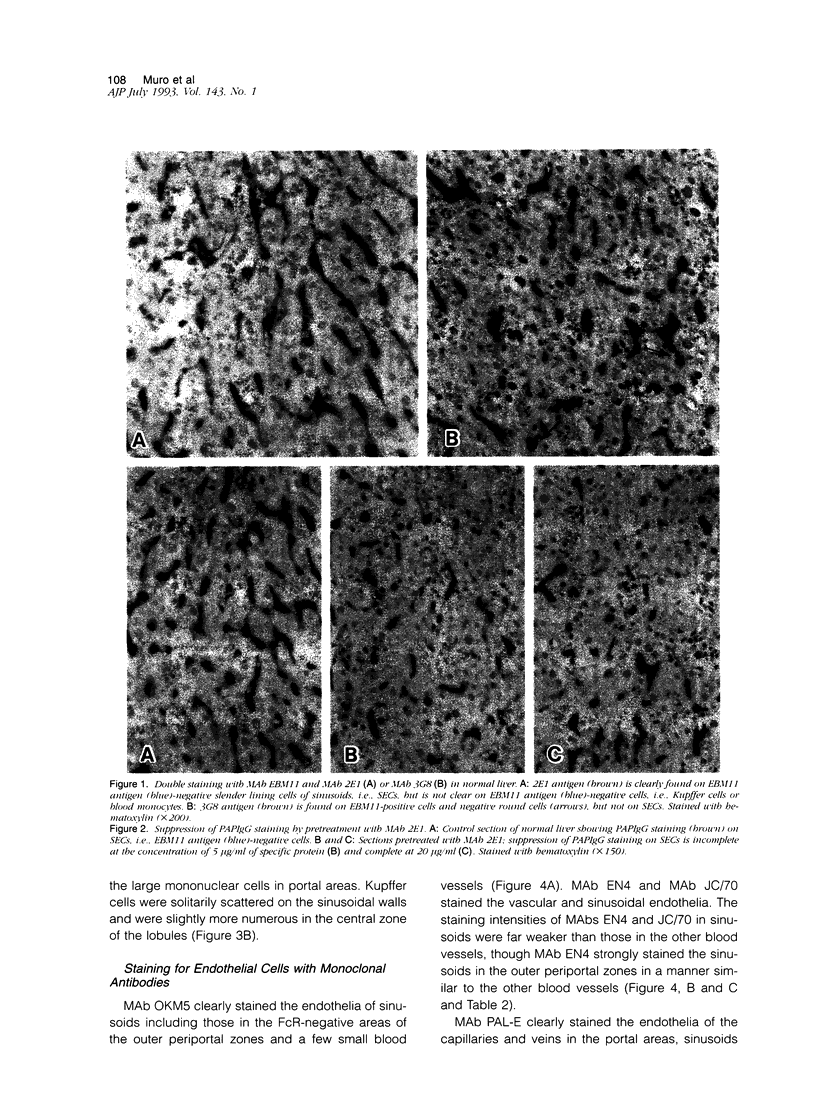
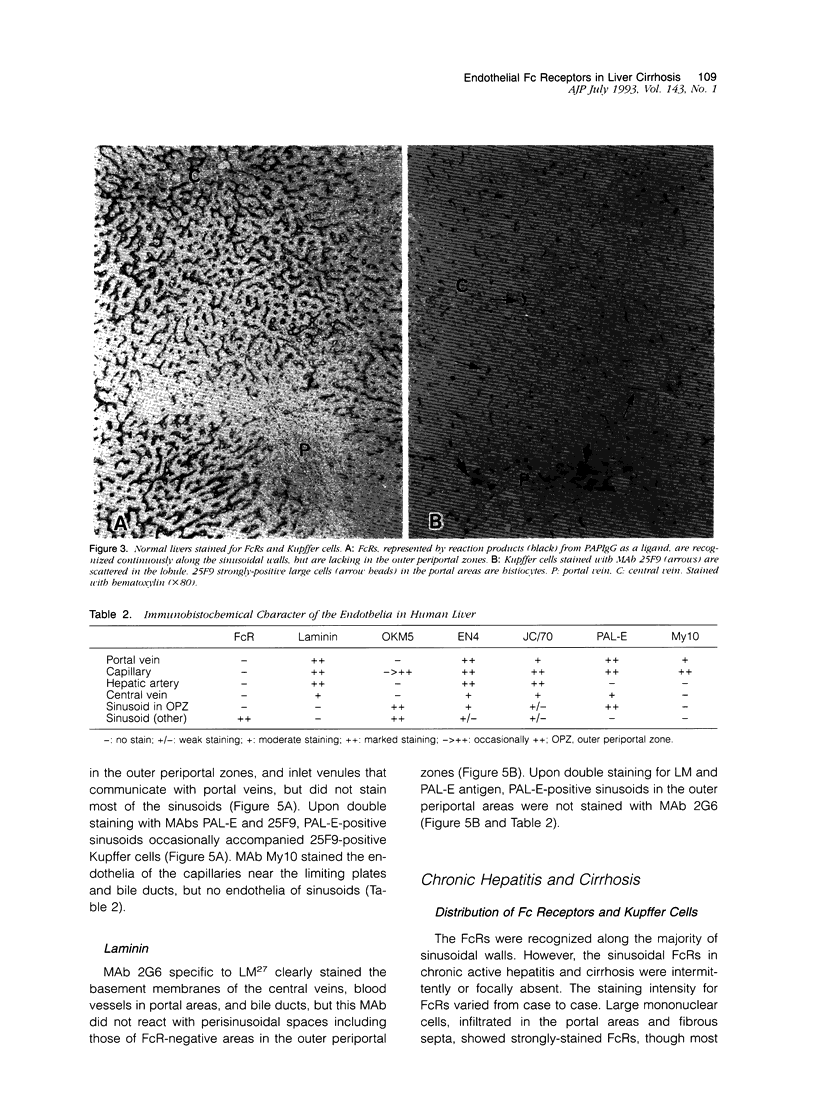
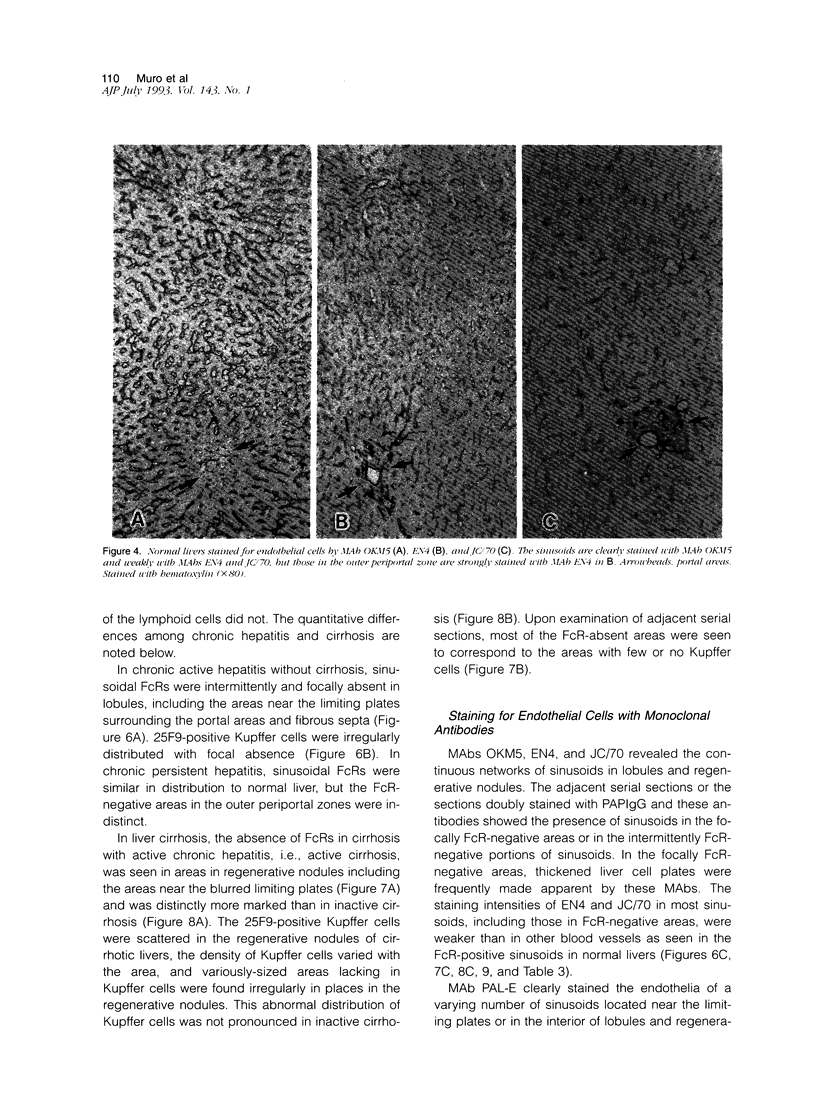
- Ito I., Muro H., Kosugi I., Shirasawa H. Alterations in Fc receptor activity in sinusoidal endothelial cells and Kupffer cells during D-galactosamine (GalN)-induced liver injury in rats. A histological study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;58(6):417–425. doi: 10.1007/BF02890101. [DOI] [PubMed] [Google Scholar]
- Kelly P. M., Bliss E., Morton J. A., Burns J., McGee J. O. Monoclonal antibody EBM/11: high cellular specificity for human macrophages. J Clin Pathol. 1988 May;41(5):510–515. doi: 10.1136/jcp.41.5.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi I., Muro H., Shirasawa H., Ito I. Endocytosis of soluble IgG immune complex and its transport to lysosomes in hepatic sinusoidal endothelial cells. J Hepatol. 1992 Sep;16(1-2):106–114. doi: 10.1016/s0168-8278(05)80102-3. [DOI] [PubMed] [Google Scholar]
- Lough J., Rosenthall L., Arzoumanian A., Goresky C. A. Kupffer cell depletion associated with capillarization of liver sinusoids in carbon tetrachloride-induced rat liver cirrhosis. J Hepatol. 1987 Oct;5(2):190–198. doi: 10.1016/s0168-8278(87)80572-x. [DOI] [PubMed] [Google Scholar]
- Maher J. J., Friedman S. L., Roll F. J., Bissell D. M. Immunolocalization of laminin in normal rat liver and biosynthesis of laminin by hepatic lipocytes in primary culture. Gastroenterology. 1988 Apr;94(4):1053–1062. doi: 10.1016/0016-5085(88)90566-5. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Delgado F. M., Amenta P. S. The extracellular matrix in hepatic regeneration. Localization of collagen types I, III, IV, laminin, and fibronectin. Lab Invest. 1991 Feb;64(2):157–166. [PubMed] [Google Scholar]
- Martinez-Hernandez A., Martinez J. The role of capillarization in hepatic failure: studies in carbon tetrachloride-induced cirrhosis. Hepatology. 1991 Nov;14(5):864–874. doi: 10.1002/hep.1840140519. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. II. Electron immunohistochemical studies in rats with CCl4-induced cirrhosis. Lab Invest. 1985 Aug;53(2):166–186. [PubMed] [Google Scholar]
- McGary C. T., Raja R. H., Weigel P. H. Endocytosis of hyaluronic acid by rat liver endothelial cells. Evidence for receptor recycling. Biochem J. 1989 Feb 1;257(3):875–884. doi: 10.1042/bj2570875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micklem K. J., Stross W. P., Willis A. C., Cordell J. L., Jones M., Mason D. Y. Different isoforms of human FcRII distinguished by CDw32 antibodies. J Immunol. 1990 Mar 15;144(6):2295–2303. [PubMed] [Google Scholar]
- Morin O., Patry P., Lafleur L. Heterogeneity of endothelial cells of adult rat liver as resolved by sedimentation velocity and flow cytometry. J Cell Physiol. 1984 Jun;119(3):327–334. doi: 10.1002/jcp.1041190311. [DOI] [PubMed] [Google Scholar]
- Muro H., Shirasawa H., Kosugi I., Ito I. Defect of sinusoidal Fc receptors and immune complex uptake in CCl4-induced liver cirrhosis in rats. Gastroenterology. 1990 Jul;99(1):200–210. doi: 10.1016/0016-5085(90)91249-6. [DOI] [PubMed] [Google Scholar]
- Muro H., Shirasawa H., Maeda M., Nakamura S. Fc receptors of liver sinusoidal endothelium in normal rats and humans. A histologic study with soluble immune complexes. Gastroenterology. 1987 Nov;93(5):1078–1085. doi: 10.1016/0016-5085(87)90572-5. [DOI] [PubMed] [Google Scholar]
- Muro H., Shirasawa H., Takahashi Y., Maeda M., Nakamura S. Localization of Fc receptors on liver sinusoidal endothelium. A histological study by electron microscopy. Acta Pathol Jpn. 1988 Mar;38(3):291–301. doi: 10.1111/j.1440-1827.1988.tb02302.x. [DOI] [PubMed] [Google Scholar]
- Parums D. V., Cordell J. L., Micklem K., Heryet A. R., Gatter K. C., Mason D. Y. JC70: a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J Clin Pathol. 1990 Sep;43(9):752–757. doi: 10.1136/jcp.43.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussia B., Trinchieri G., Jackson A., Warner N. L., Faust J., Rumpold H., Kraft D., Lanier L. L. The Fc receptor for IgG on human natural killer cells: phenotypic, functional, and comparative studies with monoclonal antibodies. J Immunol. 1984 Jul;133(1):180–189. [PubMed] [Google Scholar]
- Petrovic L. M., Burroughs A., Scheuer P. J. Hepatic sinusoidal endothelium: Ulex lectin binding. Histopathology. 1989 Mar;14(3):233–243. doi: 10.1111/j.1365-2559.1989.tb02142.x. [DOI] [PubMed] [Google Scholar]
- Potter M. Immunoglobulin-producing tumors and myeloma proteins of mice. Physiol Rev. 1972 Jul;52(3):631–719. doi: 10.1152/physrev.1972.52.3.631. [DOI] [PubMed] [Google Scholar]
- Praaning-van Dalen D. P., de Leeuw A. M., Brouwer A., Knook D. L. Rat liver endothelial cells have a greater capacity than Kupffer cells to endocytose N-acetylglucosamine- and mannose-terminated glycoproteins. Hepatology. 1987 Jul-Aug;7(4):672–679. doi: 10.1002/hep.1840070410. [DOI] [PubMed] [Google Scholar]
- Pulford K., Souhami R. L. The surface properties and antigen-presenting function of hepatic non-parenchymal cells. Clin Exp Immunol. 1981 Dec;46(3):581–588. [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Kinet J. P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- SCHAFFNER F., POPER H. Capillarization of hepatic sinusoids in man. Gastroenterology. 1963 Mar;44:239–242. [PubMed] [Google Scholar]
- Sanes J. R., Engvall E., Butkowski R., Hunter D. D. Molecular heterogeneity of basal laminae: isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J Cell Biol. 1990 Oct;111(4):1685–1699. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schena F. P., Pastore G., Pastore A., Angarano G., Meliconi L., Schiraldi O., Bonomo L. Characterization of the circulating immune complexes in acute and chronic liver diseases. J Clin Lab Immunol. 1983 Apr;10(4):179–183. [PubMed] [Google Scholar]
- Schlingemann R. O., Dingjan G. M., Emeis J. J., Blok J., Warnaar S. O., Ruiter D. J. Monoclonal antibody PAL-E specific for endothelium. Lab Invest. 1985 Jan;52(1):71–76. [PubMed] [Google Scholar]
- Scoazec J. Y., Feldmann G. In situ immunophenotyping study of endothelial cells of the human hepatic sinusoid: results and functional implications. Hepatology. 1991 Nov;14(5):789–797. doi: 10.1002/hep.1840140508. [DOI] [PubMed] [Google Scholar]
- Sedmak D. D., Davis D. H., Singh U., van de Winkel J. G., Anderson C. L. Expression of IgG Fc receptor antigens in placenta and on endothelial cells in humans. An immunohistochemical study. Am J Pathol. 1991 Jan;138(1):175–181. [PMC free article] [PubMed] [Google Scholar]
- Skogh T., Blomhoff R., Eskild W., Berg T. Hepatic uptake of circulating IgG immune complexes. Immunology. 1985 Aug;55(4):585–594. [PMC free article] [PubMed] [Google Scholar]
- Smedsrød B., Pertoft H., Gustafson S., Laurent T. C. Scavenger functions of the liver endothelial cell. Biochem J. 1990 Mar 1;266(2):313–327. doi: 10.1042/bj2660313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H. C., De Villiers D., Potter B., Hodgson H., Jain S., Jewell D. P., Sherlock S. Immune complexes in acute and chronic liver disease. Clin Exp Immunol. 1978 Feb;31(2):150–157. [PMC free article] [PubMed] [Google Scholar]
- Thomas H. C., McSween R. N., White R. G. Role of the liver in controlling the immunogenicity of commensal bacteria in the gut. Lancet. 1973 Jun 9;1(7815):1288–1291. doi: 10.1016/s0140-6736(73)91300-7. [DOI] [PubMed] [Google Scholar]
- Volpes R., van den Oord J. J., Desmet V. J. Adhesive molecules in liver disease. Immunohistochemical distribution of thrombospondin receptors in chronic HBV infection. J Hepatol. 1990 May;10(3):297–304. doi: 10.1016/0168-8278(90)90136-f. [DOI] [PubMed] [Google Scholar]
- Watt S. M., Karhi K., Gatter K., Furley A. J., Katz F. E., Healy L. E., Altass L. J., Bradley N. J., Sutherland D. R., Levinsky R. Distribution and epitope analysis of the cell membrane glycoprotein (HPCA-1) associated with human hemopoietic progenitor cells. Leukemia. 1987 May;1(5):417–426. [PubMed] [Google Scholar]
- Wewer U. M., Engvall E., Paulsson M., Yamada Y., Albrechtsen R. Laminin A, B1, B2, S and M subunits in the postnatal rat liver development and after partial hepatectomy. Lab Invest. 1992 Mar;66(3):378–389. [PubMed] [Google Scholar]
- Zwadlo G., Bröcker E. B., von Bassewitz D. B., Feige U., Sorg C. A monoclonal antibody to a differentiation antigen present on mature human macrophages and absent from monocytes. J Immunol. 1985 Mar;134(3):1487–1492. [PubMed] [Google Scholar]
- van der Laan-Klamer S. M., Harms G., Atmosoerodjo J. E., Meijer D. K., Hardonk M. J., Hoedemaeker P. J. Studies on the mechanism of binding and uptake of immune complexes by various cell types of rat liver in vivo. Scand J Immunol. 1986 Jan;23(1):127–133. doi: 10.1111/j.1365-3083.1986.tb01950.x. [DOI] [PubMed] [Google Scholar]