Abstract
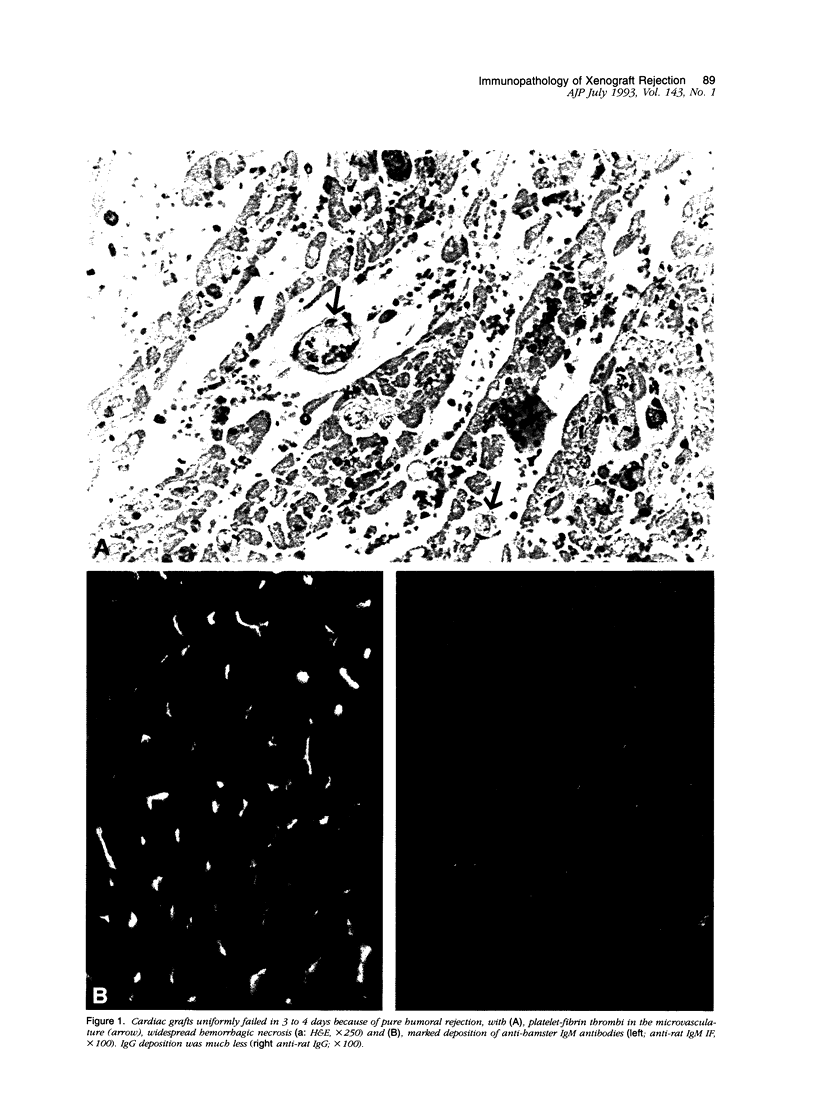
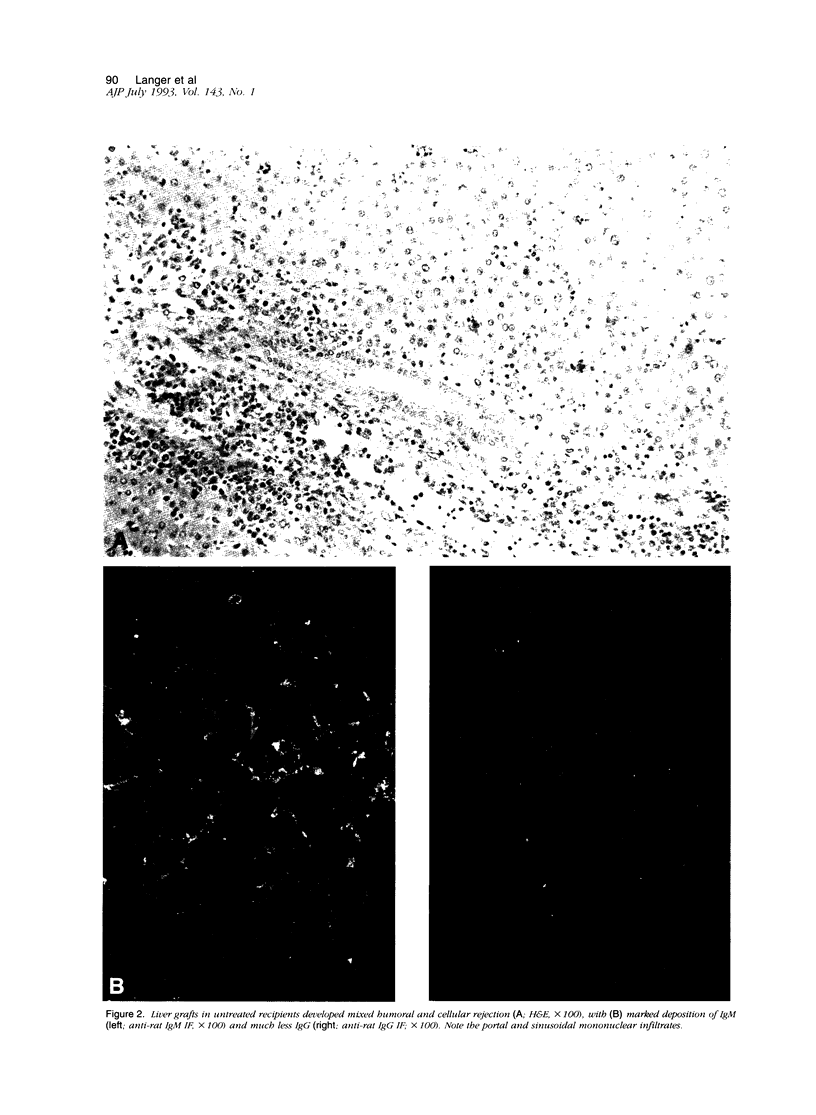
Normal Lewis rat serum contains antibodies (IgM > IgG) that bind to hamster leukocytes and endothelial cells. Transplantation of either the heart or liver from hamster rat results in release of hamster hematolymphoid cells from the graft, which lodge in the recipient spleen (cell migration), where recipient T- and B-cell populations initiate DNA synthesis within one day. There is marked stimulation of splenic IgM++(bright)/IgD+(dull) B cells in the marginal zone and red pulp, which account for 48% of the total splenic blast cell population by 4 days after liver transplantation. CD4+ predominant T-cell proliferation in the splenic periarterial lymphatic sheath and paracortex of peripheral lymph nodes occurs almost simultaneously. The effector phase of rejection in cardiac recipients is dominated by complement-fixing IgM antibodies, which increase daily and result in graft destruction in 3 to 4 days, even in animals treated with FK506. In liver recipients, combined antibody and cellular rejection, associated with graft infiltration by OX8+ natural killer, and fewer W3/25+ (CD4) lymphocytes, are responsible for graft failure in untreated recipients at 6 to 7 days. FK506 inhibits the T-cell response in liver recipients and significantly prolongs graft survival, but does not prevent the rise or deposition of IgM antibodies in the graft. However, a single injection of cyclophosphamide 10 days before transplantation effectively depletes the splenic IgM++(bright)/Ig+(dull) cells and in combination with FK506, results in 100% survival of both cardiac and hepatic xenografts for more than 60 days. Although extrapolation of morphological findings to functional significance is fraught with potential problems, we propose the following mechanisms of xenografts rejection. The reaction initially appears to involve primitive host defense mechanisms, including an IgM-producing subpopulation of splenic B cells and natural killer cells. Based on the reaction and distribution of OX8+ and W3/25+ cells, antibody-dependent cell cytotoxicity and delayed-type hypersensitivity responses seem worthy of further investigation as possible effector mechanisms. Effective control of xenograft rejection is likely to require a dual pharmaceutical approach, one to contain T-cell immunity and another to blunt the primitive B-cell response.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach F. H., Turman M. A., Vercellotti G. M., Platt J. L., Dalmasso A. P. Accommodation: a working paradigm for progressing toward clinical discordant xenografting. Transplant Proc. 1991 Feb;23(1 Pt 1):205–207. [PubMed] [Google Scholar]
- Bazin H., Gray D., Platteau B., MacLennan I. C. Distinct delta + and delta - B-lymphocyte lineages in the rat. Ann N Y Acad Sci. 1982;399:157–174. doi: 10.1111/j.1749-6632.1982.tb25671.x. [DOI] [PubMed] [Google Scholar]
- Bazin H., Platteau B., Beckers A., Pauwels R. Differential effect of neonatal injections of anti-mu or anti-delta antibodies on the synthesis of IgM, IgD, IgE, IgA, IgG1, IgG2a, IgG2b, and IgG2c immunoglobulin classes. J Immunol. 1978 Nov;121(5):2083–2087. [PubMed] [Google Scholar]
- Briles D. E., Claflin J. L., Schroer K., Forman C. Mouse Igg3 antibodies are highly protective against infection with Streptococcus pneumoniae. Nature. 1981 Nov 5;294(5836):88–90. doi: 10.1038/294088a0. [DOI] [PubMed] [Google Scholar]
- Carobbi A., Araneda D., Patselas T., Thomas J., Mosca F., Thomas F. Effect of splenectomy in combination with FK 506 and 15-deoxyspergualin on cardiac xenograft survival. Transplant Proc. 1991 Feb;23(1 Pt 1):549–550. [PubMed] [Google Scholar]
- Cooper D. K., Human P. A., Rose A. G., Rees J., Keraan M., Reichart B., Du Toit E., Oriol R. The role of ABO blood group compatibility in heart transplantation between closely related animal species. An experimental study using the vervet monkey to baboon cardiac xenograft model. J Thorac Cardiovasc Surg. 1989 Mar;97(3):447–455. [PubMed] [Google Scholar]
- Cramer D. V., Chapman F. A., Jaffee B. D., Zajac I., Hreha-Eiras G., Yasunaga C., Wu G. D., Makowka L. The prolongation of concordant hamster-to-rat cardiac xenografts by brequinar sodium. Transplantation. 1992 Sep;54(3):403–408. doi: 10.1097/00007890-199209000-00003. [DOI] [PubMed] [Google Scholar]
- Demetris A. J., Qian S., Sun H., Fung J. J., Yagihashi A., Murase N., Iwaki Y., Gambrell B., Starzl T. E. Early events in liver allograft rejection. Delineation of sites of simultaneous intragraft and recipient lymphoid tissue sensitization. Am J Pathol. 1991 Mar;138(3):609–618. [PMC free article] [PubMed] [Google Scholar]
- Der Balian G. P., Slack J., Clevinger B. L., Bazin H., Davie J. M. Subclass restriction of murine antibodies. III. Antigens that stimulate IgG3 in mice stimulate IgG2c in rats. J Exp Med. 1980 Jul 1;152(1):209–218. doi: 10.1084/jem.152.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D., MacLennan I. C., Bazin H., Khan M. Migrant mu+ delta+ and static mu+ delta- B lymphocyte subsets. Eur J Immunol. 1982 Jul;12(7):564–569. doi: 10.1002/eji.1830120707. [DOI] [PubMed] [Google Scholar]
- Hasan R., Van den Bogaerde J. B., Wallwork J., White D. J. Evidence that long-term survival of concordant xenografts is achieved by inhibition of antispecies antibody production. Transplantation. 1992 Sep;54(3):408–413. doi: 10.1097/00007890-199209000-00004. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Lalor P. A., Sidman C., Moore W. A., Parks D. R., Herzenberg L. A. The Ly-1 B cell lineage. Immunol Rev. 1986 Oct;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992 Jan;13(1):11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- Jongen P. J., Joosten E. M., Berden J. H., Gabreëls-Festen A. A. Cyclosporine therapy in chronic inflammatory demyelinating polyradiculoneuropathy--a preliminary report of clinical results in two patients. Transplant Proc. 1988 Jun;20(3 Suppl 4):329–332. [PubMed] [Google Scholar]
- Kamada N., Calne R. Y. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979 Jul;28(1):47–50. [PubMed] [Google Scholar]
- Kroese F. G., Butcher E. C., Lalor P. A., Stall A. M., Herzenberg L. A. The rat B cell system: the anatomical localization of flow cytometry-defined B cell subpopulations. Eur J Immunol. 1990 Jul;20(7):1527–1534. doi: 10.1002/eji.1830200718. [DOI] [PubMed] [Google Scholar]
- Kumararatne D. S., Bazin H., MacLennan I. C. Marginal zones: the major B cell compartment of rat spleens. Eur J Immunol. 1981 Nov;11(11):858–864. doi: 10.1002/eji.1830111103. [DOI] [PubMed] [Google Scholar]
- Kumararatne D. S., Gagnon R. F., Smart Y. Selective loss of large lymphocytes from the marginal zone of the white pulp in rat spleens following a single dose of cyclophosphamide. A study using quantitative histological methods. Immunology. 1980 May;40(1):123–131. [PMC free article] [PubMed] [Google Scholar]
- Kumararatne D. S., MacLennan I. C. Cells of the marginal zone of the spleen are lymphocytes derived from recirculating precursors. Eur J Immunol. 1981 Nov;11(11):865–869. doi: 10.1002/eji.1830111104. [DOI] [PubMed] [Google Scholar]
- Larsen C. P., Morris P. J., Austyn J. M. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990 Jan 1;171(1):307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. M., Li S. Q., Wee A., Chong S. M., Hu C., Rauff A., White D. J. Both concordant and discordant heart xenografts are rejected by athymic (nude) rats with the same tempo as in T cell competent animals. Transplant Proc. 1991 Feb;23(1 Pt 1):581–582. [PubMed] [Google Scholar]
- Liu Y. J., Zhang J., Lane P. J., Chan E. Y., MacLennan I. C. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991 Dec;21(12):2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- Monden M., Valdivia L. A., Gotoh M., Hasuike Y., Kubota N., Kanai T., Okamura J., Mori T. Hamster-to-rat orthotopic liver xenografts. Transplantation. 1987 May;43(5):745–746. [PubMed] [Google Scholar]
- Ono K., Lindsey E. S. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969 Feb;57(2):225–229. [PubMed] [Google Scholar]
- Platt J. L., Lindman B. J., Chen H., Spitalnik S. L., Bach F. H. Endothelial cell antigens recognized by xenoreactive human natural antibodies. Transplantation. 1990 Nov;50(5):817–822. doi: 10.1097/00007890-199011000-00015. [DOI] [PubMed] [Google Scholar]
- Putnam C. W., Halgrimson C. G., Groth C. G., Kashiwagi N., Porter K. A., Starzl T. E. Immunosuppression with cyclophosphamide in the dog. Clin Exp Immunol. 1975 Nov;22(2):323–329. [PMC free article] [PubMed] [Google Scholar]
- Reichenspurner H., Human P. A., Boehm D. H., Rose A. G., May R., Cooper D. K., Zilla P., Reichart B. Optimalization of immunosuppression after xenogeneic heart transplantation in primates. J Heart Transplant. 1989 May-Jun;8(3):200–208. [PubMed] [Google Scholar]
- Ron Y., De Baetselier P., Segal S. Involvement of the spleen in murine B cell differentiation. Eur J Immunol. 1981 Feb;11(2):94–99. doi: 10.1002/eji.1830110206. [DOI] [PubMed] [Google Scholar]
- Starzl T. E., Demetris A. J., Murase N., Ildstad S., Ricordi C., Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992 Jun 27;339(8809):1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Halgrimson C. G., Penn I., Martineau G., Schroter G., Amemiya H., Putnam C. W., Groth C. G. Cyclophosphamide and human organ transplantation. Lancet. 1971 Jul 10;2(7715):70–74. doi: 10.1016/s0140-6736(71)92046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzl T. E., Putnam C. W., Halgrimson C. G., Schroter G. T., Martineau G., Launois B., Corman J. L., Penn I., Booth A. S., Jr, Groth C. G. Cyclophosphamide and whole organ transplantation in human beings. Surg Gynecol Obstet. 1971 Dec;133(6):981–991. [PMC free article] [PubMed] [Google Scholar]
- Todo S., Tzakis A. G., Abu-Elmagd K., Reyes J., Nakamura K., Casavilla A., Selby R., Nour B. M., Wright H., Fung J. J. Intestinal transplantation in composite visceral grafts or alone. Ann Surg. 1992 Sep;216(3):223–234. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turman M. A., Casali P., Notkins A. L., Bach F. H., Platt J. L. Polyreactivity and antigen specificity of human xenoreactive monoclonal and serum natural antibodies. Transplantation. 1991 Oct;52(4):710–717. doi: 10.1097/00007890-199110000-00024. [DOI] [PubMed] [Google Scholar]
- Valdivia L. A., Fung J. J., Demetris A. J., Starzl T. E. Differential survival of hamster-to-rat liver and cardiac xenografts under FK 506 immunosuppression. Transplant Proc. 1991 Dec;23(6):3269–3271. [PMC free article] [PubMed] [Google Scholar]
- Valdivia L. A., Monden M., Gotoh M., Hasuike Y., Kubota N., Ichikawa T., Okamura J., Mori T. Prolonged survival of hamster-to-rat liver xenografts using splenectomy and cyclosporine administration. Transplantation. 1987 Dec;44(6):759–763. doi: 10.1097/00007890-198712000-00007. [DOI] [PubMed] [Google Scholar]
- Wolf H. K., Michalopoulos G. K. Proliferating cell nuclear antigen in human placenta and trophoblastic disease. Pediatr Pathol. 1992 Mar-Apr;12(2):147–154. doi: 10.3109/15513819209023291. [DOI] [PubMed] [Google Scholar]
- van den Bogaerde J., Aspinall R., Wang M. W., Cary N., Lim S., Wright L., White D. Induction of long-term survival of hamster heart xenografts in rats. Transplantation. 1991 Jul;52(1):15–20. doi: 10.1097/00007890-199107000-00003. [DOI] [PubMed] [Google Scholar]