Abstract
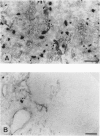
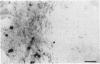
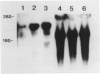
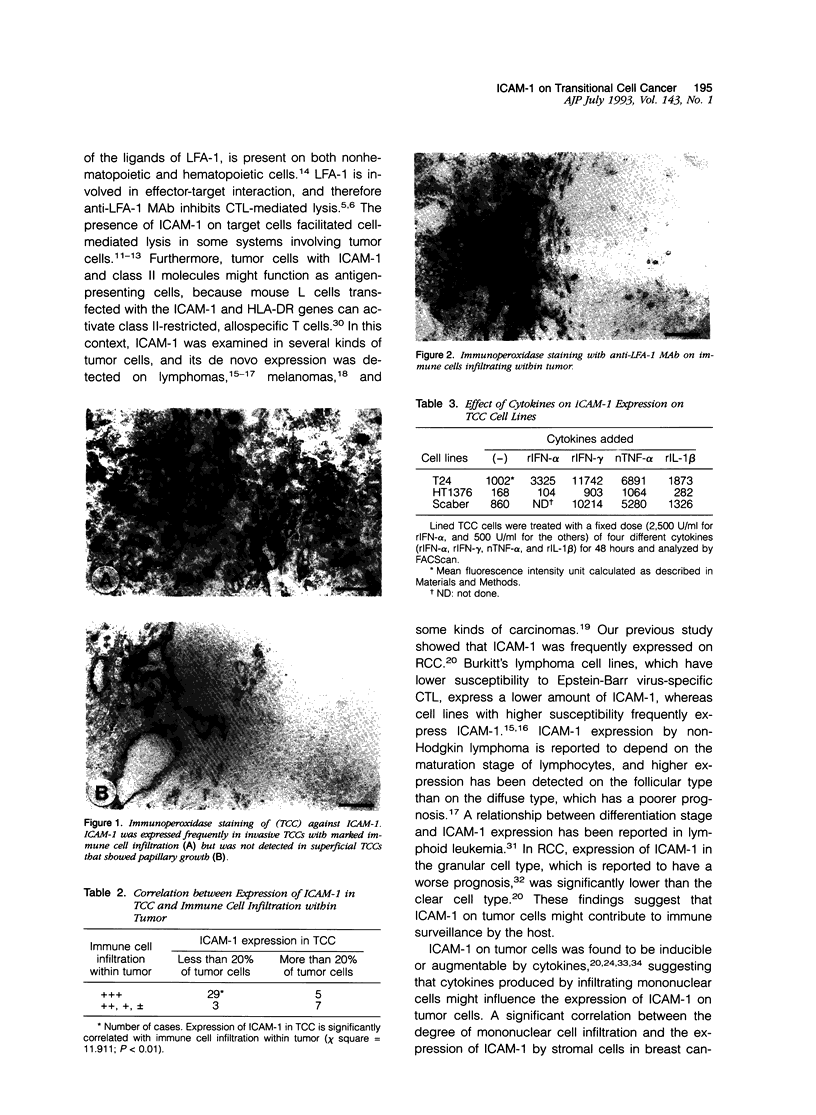
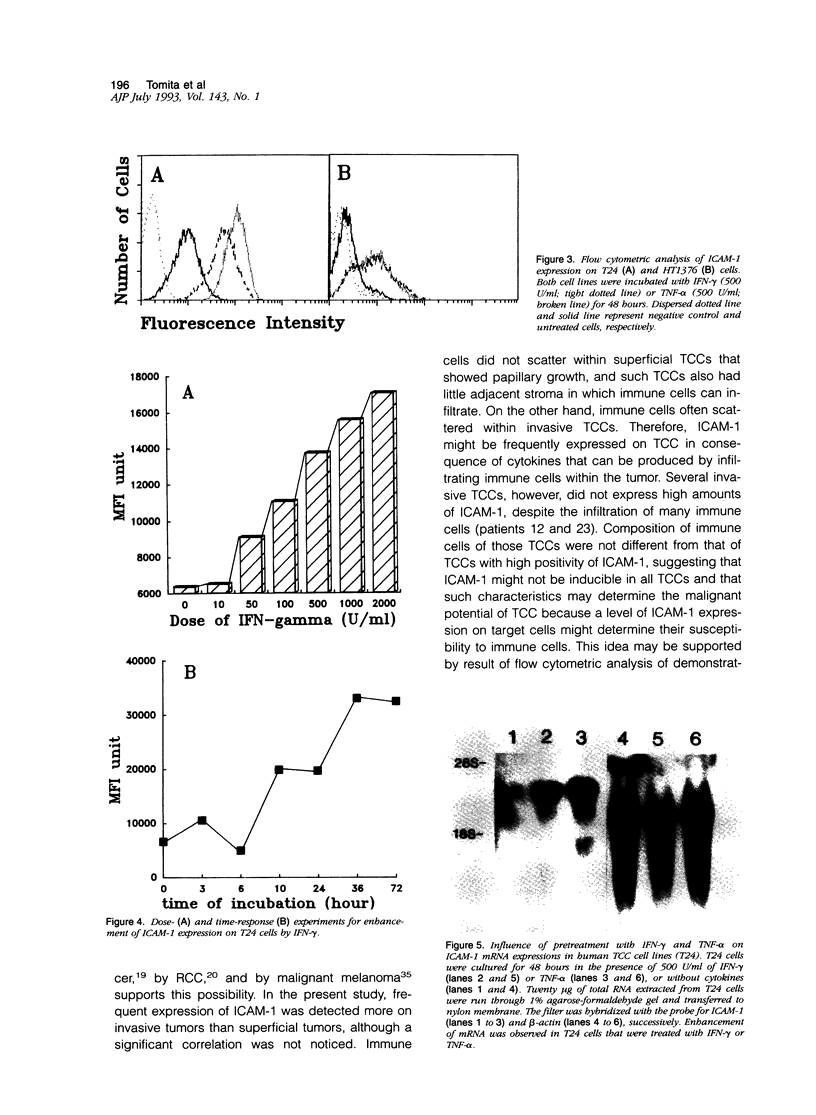
Immunohistochemical examination demonstrated expression of intercellular adhesion molecule-1 (ICAM-1) on 17 of 44 transitional cell cancers (TCCs) but not on normal transitional cells. ICAM-1 was frequently expressed in higher stage tumors, especially in those with abundant immune cells scattered within tumor. Analysis of infiltrating immune cells showed that they were composed mainly of T lymphocytes and a smaller number of macrophages bearing the lymphocyte function-associated antigen-1 (LFA-1). Expression of ICAM-1 on transitional cell cancer cell lines was augmented by in vitro treatment with interferon-gamma, tumor necrosis factor-alpha, and interleukin-1 beta. Furthermore, Northern blot analysis revealed higher quantities of a 3.3-kb RNA in T24 cells exposed to interferon-gamma or tumor necrosis factor-alpha. These results suggest that the expression of ICAM-1 on transitional cell cancers might be modified by cytokines produced by infiltrating immune cells, which might facilitate immune responses against cancer cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann D. M., Hogg N., Trowsdale J., Wilkinson D. Cotransfection of ICAM-1 and HLA-DR reconstitutes human antigen-presenting cell function in mouse L cells. Nature. 1989 Apr 6;338(6215):512–514. doi: 10.1038/338512a0. [DOI] [PubMed] [Google Scholar]
- Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981 Jun 12;212(4500):1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- Billaud M., Calender A., Seigneurin J. M., Lenoir G. M. LFA-1, LFA-3, and ICAM-1 expression in Burkitt's lymphoma. Lancet. 1987 Dec 5;2(8571):1327–1328. doi: 10.1016/s0140-6736(87)91214-1. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Braakman E., Goedegebuure P. S., Vreugdenhil R. J., Segal D. M., Shaw S., Bolhuis R. L. ICAM- melanoma cells are relatively resistant to CD3-mediated T-cell lysis. Int J Cancer. 1990 Sep 15;46(3):475–480. doi: 10.1002/ijc.2910460325. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Goedegebuure P. S., Braakman E., Segal D. M., Vreugdenhil R. J., Bolhuis R. L. Lymphocyte leukocyte function-associated antigen 1 interacting with target cell intercellular adhesion molecule 1 co-activates cytolysis triggered via CD16 or the receptor involved in major histocompatibility antigen-unrestricted lysis. Int Immunol. 1990;2(12):1213–1220. doi: 10.1093/intimm/2.12.1213. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Murray R. J., Edwards C. F., Rickinson A. B. Downregulation of cell adhesion molecules LFA-3 and ICAM-1 in Epstein-Barr virus-positive Burkitt's lymphoma underlies tumor cell escape from virus-specific T cell surveillance. J Exp Med. 1988 Jun 1;167(6):1811–1824. doi: 10.1084/jem.167.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarini L., Temponi M., Bruce J. N., Bollon A. P., Duigou G. J., Moulton T. A., Ferrone S., Fisher P. B. Expression and modulation by cytokines of the intercellular adhesion molecule-1 (ICAM-1) in human central nervous system tumor cell cultures. Int J Cancer. 1990 Dec 15;46(6):1041–1047. doi: 10.1002/ijc.2910460616. [DOI] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T. A novel repeated element with Z-DNA-forming potential is widely found in evolutionarily diverse eukaryotic genomes. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6465–6469. doi: 10.1073/pnas.79.21.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildreth J. E., Gotch F. M., Hildreth P. D., McMichael A. J. A human lymphocyte-associated antigen involved in cell-mediated lympholysis. Eur J Immunol. 1983 Mar;13(3):202–208. doi: 10.1002/eji.1830130305. [DOI] [PubMed] [Google Scholar]
- Johnson J. P., Stade B. G., Holzmann B., Schwäble W., Riethmüller G. De novo expression of intercellular-adhesion molecule 1 in melanoma correlates with increased risk of metastasis. Proc Natl Acad Sci U S A. 1989 Jan;86(2):641–644. doi: 10.1073/pnas.86.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krensky A. M., Sanchez-Madrid F., Robbins E., Nagy J. A., Springer T. A., Burakoff S. J. The functional significance, distribution, and structure of LFA-1, LFA-2, and LFA-3: cell surface antigens associated with CTL-target interactions. J Immunol. 1983 Aug;131(2):611–616. [PubMed] [Google Scholar]
- MURPHY G. P., MOSTOFI F. K. THE SIGNIFICANCE OF CYTOPLASMIC GRANULARITY IN THE PROGNOSIS OF RENAL CELL CARCINOMA. J Urol. 1965 Jul;94:48–54. doi: 10.1016/S0022-5347(17)63566-3. [DOI] [PubMed] [Google Scholar]
- Maio M., Pinto A., Carbone A., Zagonel V., Gloghini A., Marotta G., Cirillo D., Colombatti A., Ferrara F., Del Vecchio L. Differential expression of CD54/intercellular adhesion molecule-1 in myeloid leukemias and in lymphoproliferative disorders. Blood. 1990 Aug 15;76(4):783–790. [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Dustin M. L., Springer T. A., Clark E. A., Mannoni P., Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988 Jan 7;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Gugel E. A., Dustin M. L., Springer T. A., Shaw S. Functional evidence that intercellular adhesion molecule-1 (ICAM-1) is a ligand for LFA-1-dependent adhesion in T cell-mediated cytotoxicity. Eur J Immunol. 1988 Apr;18(4):637–640. doi: 10.1002/eji.1830180423. [DOI] [PubMed] [Google Scholar]
- Miedema F., Tetteroo P. A., Hesselink W. G., Werner G., Spits H., Melief C. J. Both Fc receptors and lymphocyte-function-associated antigen 1 on human T gamma lymphocytes are required for antibody-dependent cellular cytotoxicity (killer cell activity). Eur J Immunol. 1984 Jun;14(6):518–523. doi: 10.1002/eji.1830140607. [DOI] [PubMed] [Google Scholar]
- Mortarini R., Belli F., Parmiani G., Anichini A. Cytokine-mediated modulation of HLA-class II, ICAM-1, LFA-3 and tumor-associated antigen profile of melanoma cells. Comparison with anti-proliferative activity by rIL1-beta, rTNF-alpha, rIFN-gamma, rIL4 and their combinations. Int J Cancer. 1990 Feb 15;45(2):334–341. doi: 10.1002/ijc.2910450221. [DOI] [PubMed] [Google Scholar]
- Natali P., Nicotra M. R., Cavaliere R., Bigotti A., Romano G., Temponi M., Ferrone S. Differential expression of intercellular adhesion molecule 1 in primary and metastatic melanoma lesions. Cancer Res. 1990 Feb 15;50(4):1271–1278. [PubMed] [Google Scholar]
- Rothlein R., Czajkowski M., O'Neill M. M., Marlin S. D., Mainolfi E., Merluzzi V. J. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. Regulation by pharmacologic agents and neutralizing antibodies. J Immunol. 1988 Sep 1;141(5):1665–1669. [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Schmidt R. E., Bartley G., Levine H., Schlossman S. F., Ritz J. Functional characterization of LFA-1 antigens in the interaction of human NK clones and target cells. J Immunol. 1985 Aug;135(2):1020–1025. [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Stauder R., Greil R., Schulz T. F., Thaler J., Gattringer C., Radaskiewicz T., Dierich M. P., Huber H. Expression of leucocyte function-associated antigen-1 and 7F7-antigen, an adhesion molecule related to intercellular adhesion molecule-1 (ICAM-1) in non-Hodgkin lymphomas and leukaemias: possible influence on growth pattern and leukaemic behaviour. Clin Exp Immunol. 1989 Aug;77(2):234–238. [PMC free article] [PubMed] [Google Scholar]
- Temponi M., Romano G., D'Urso C. M., Wang Z., Kekish U., Ferrone S. Profile of intercellular adhesion molecule-1 (ICAM-1) synthesized by human melanoma cell lines. Semin Oncol. 1988 Dec;15(6):595–607. [PubMed] [Google Scholar]
- Tokino T., Takahashi E., Mori M., Tanigami A., Glaser T., Park J. W., Jones C., Hori T., Nakamura Y. Isolation and mapping of 62 new RFLP markers on human chromosome 11. Am J Hum Genet. 1991 Feb;48(2):258–268. [PMC free article] [PubMed] [Google Scholar]
- Tomita Y., Nishiyama T., Fujiwara M., Sato S. Immunohistochemical detection of major histocompatibility complex antigens and quantitative analysis of tumour-infiltrating mononuclear cells in renal cell cancer. Br J Cancer. 1990 Sep;62(3):354–359. doi: 10.1038/bjc.1990.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita Y., Nishiyama T., Watanabe H., Fujiwara M., Sato S. Expression of intercellular adhesion molecule-1 (ICAM-1) on renal-cell cancer: possible significance in host immune responses. Int J Cancer. 1990 Dec 15;46(6):1001–1006. doi: 10.1002/ijc.2910460609. [DOI] [PubMed] [Google Scholar]
- Tsujisaki M., Imai K., Hirata H., Hanzawa Y., Masuya J., Nakano T., Sugiyama T., Matsui M., Hinoda Y., Yachi A. Detection of circulating intercellular adhesion molecule-1 antigen in malignant diseases. Clin Exp Immunol. 1991 Jul;85(1):3–8. doi: 10.1111/j.1365-2249.1991.tb05673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogetseder W., Feichtinger H., Schulz T. F., Schwaeble W., Tabaczewski P., Mitterer M., Böck G., Marth C., Dapunt O., Mikuz G. Expression of 7F7-antigen, a human adhesion molecule identical to intercellular adhesion molecule-1 (ICAM-1) in human carcinomas and their stromal fibroblasts. Int J Cancer. 1989 May 15;43(5):768–773. doi: 10.1002/ijc.2910430504. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- van Vreeswijk H., Ruiter D. J., Bröcker E. B., Welvaart K., Ferrone S. Differential expression of HLA-DR, DQ, and DP antigens in primary and metastatic melanoma. J Invest Dermatol. 1988 May;90(5):755–760. doi: 10.1111/1523-1747.ep12560951. [DOI] [PubMed] [Google Scholar]