Abstract
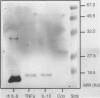
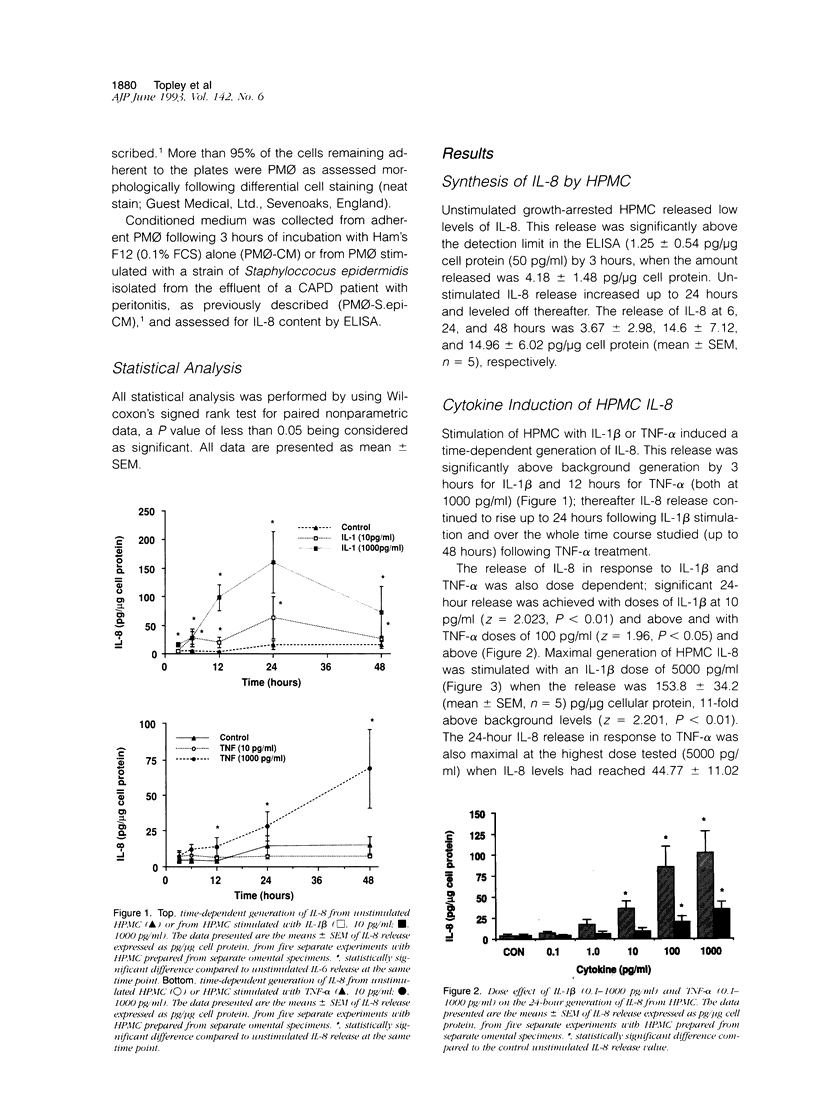
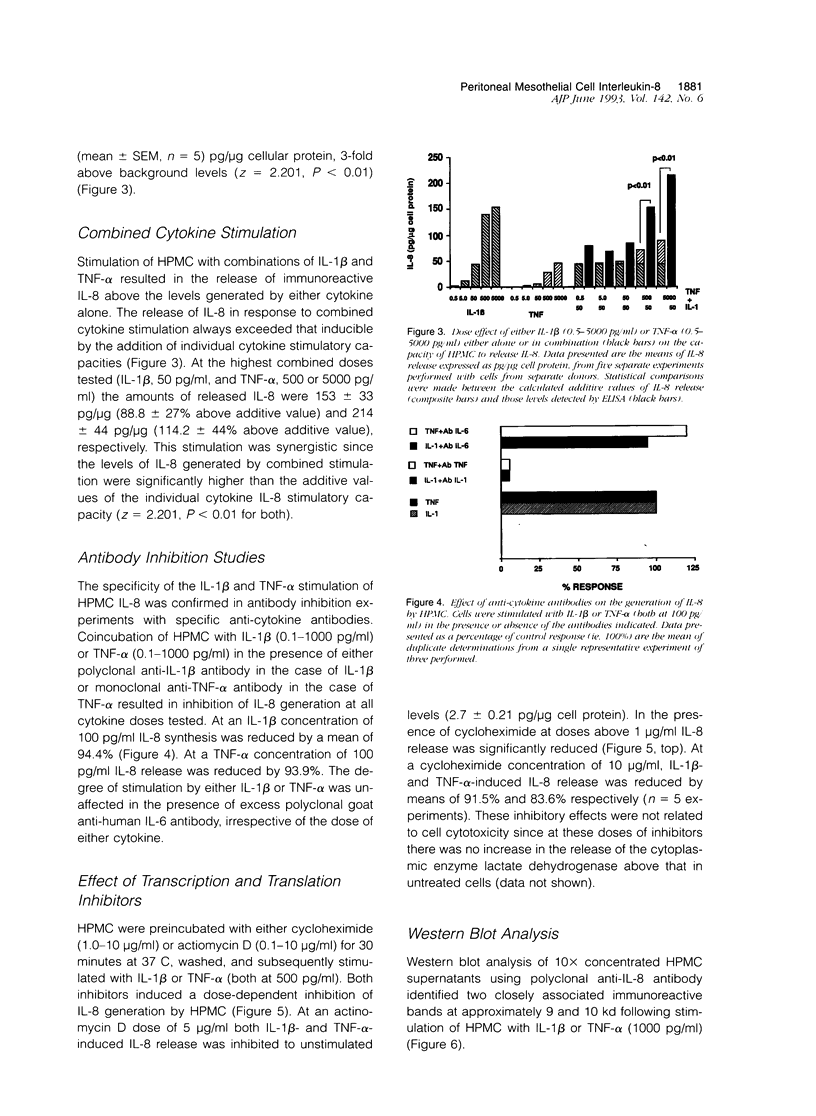
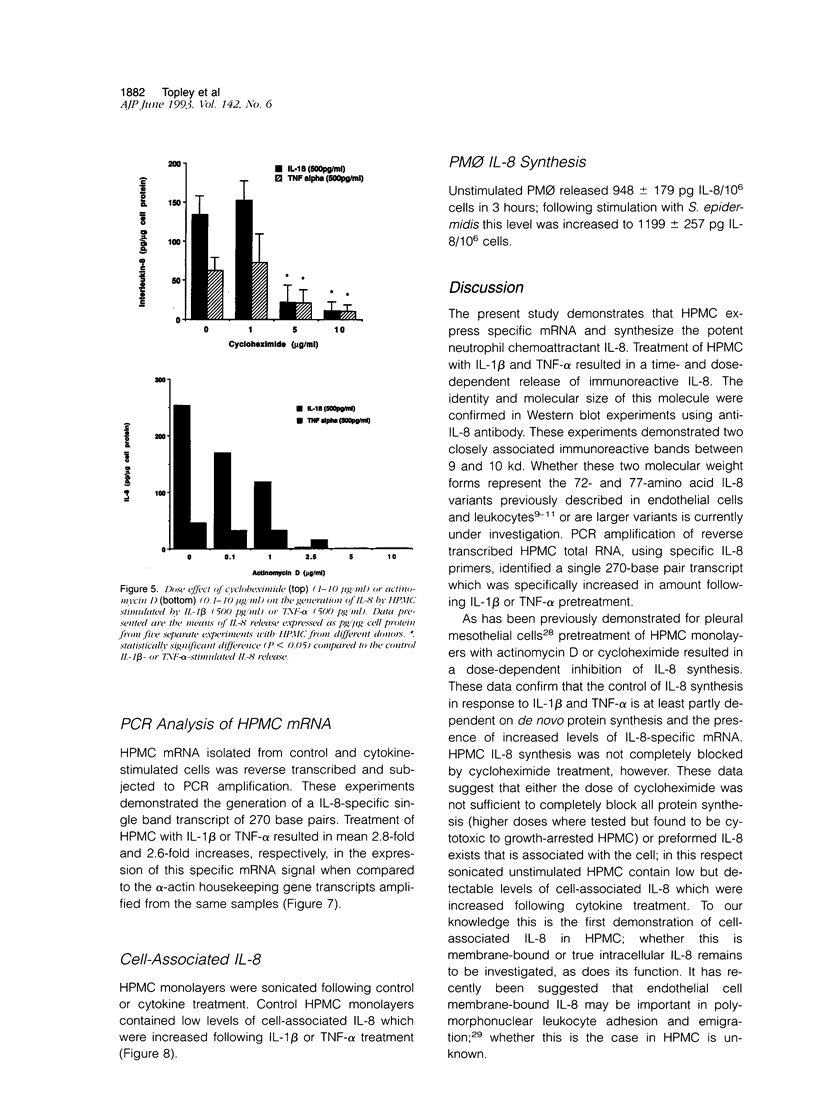
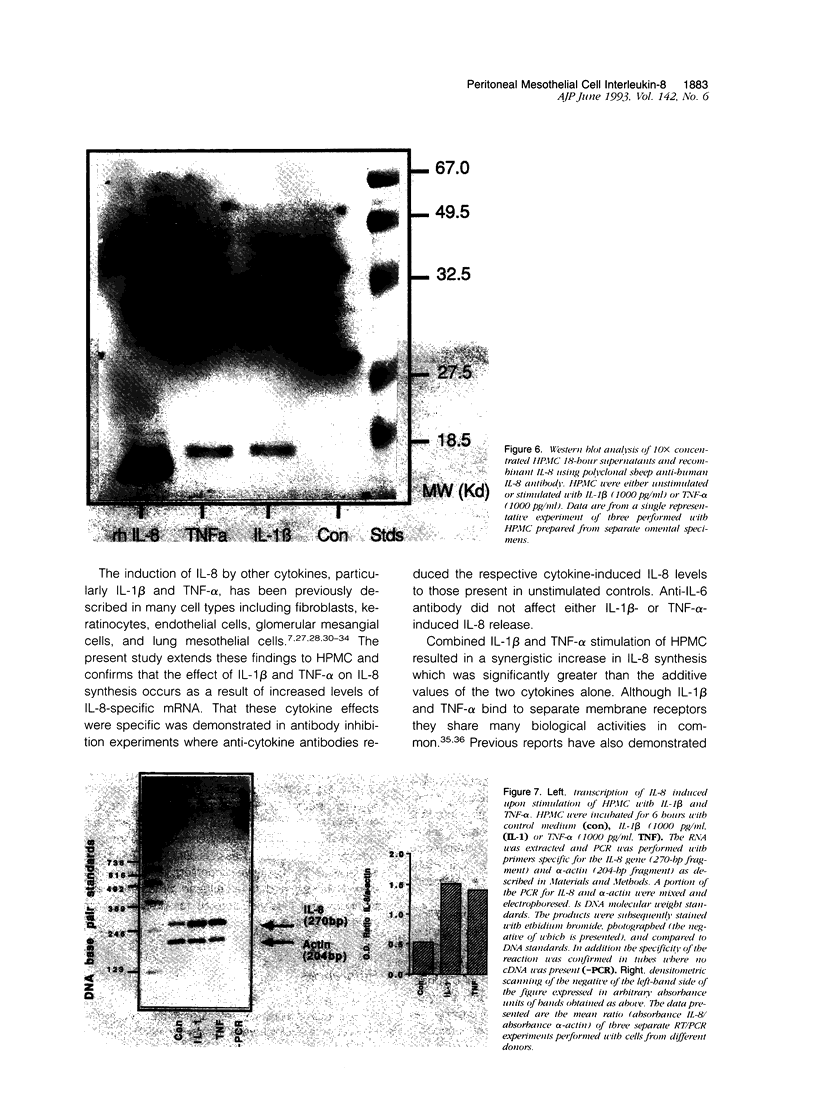
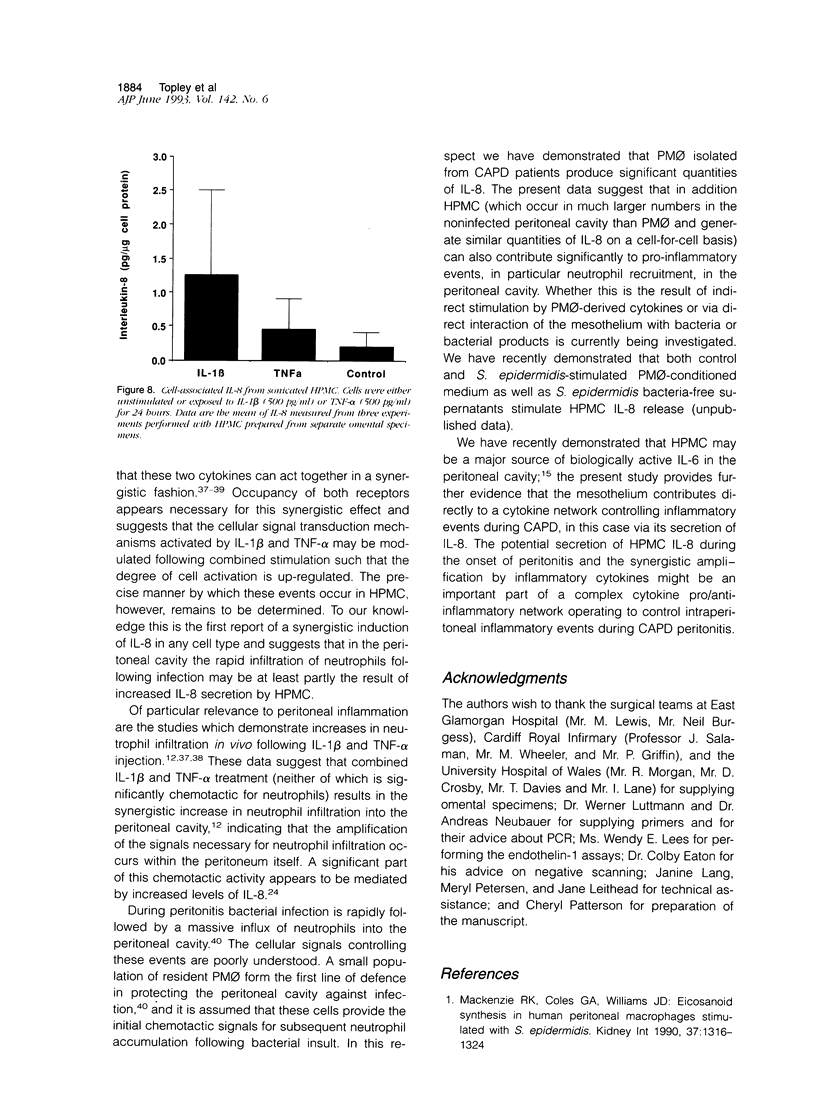
The present study demonstrates the synthesis and secretion of the neutrophil-activating peptide/interleukin-8 (IL-8) by cultured human peritoneal mesothelial cells (HPMC) and examines the regulation of its production by other cytokines. Unstimulated HPMC under growth-arrested conditions released IL-8 in a constitutive and time-dependent manner. Stimulation of HPMC with IL-1 beta or TNF-alpha resulted in a time- and dose-dependent IL-8 generation; after 24 hours the levels induced by IL-1 beta and TNF-alpha (both at 1000 pg/ml) were (mean +/- SEM, n = 5) 101 +/- 26.6 (z = 2.023; P < 0.01) and 35 +/- 8.09 (z = 2.023; P < 0.01) respectively. This release was inhibited following coincubation with the relevant anti-cytokine antibody or preincubation with either cycloheximide or actinomycin D. Treatment of HPMC with IL-1 beta or TNF-alpha resulted in increased levels of IL-8-specific mRNA. Stimulation of HPMC with combinations of IL-1 beta and TNF-alpha resulted in a synergistic increase in IL-8 release. This effect was significant at combined doses of IL-1 beta (50 pg/ml) and TNF-alpha (500 pg/ml) and above, when the release of IL-8 was 88 +/- 27% above the additive IL-8 release values (z = 2.201; P < 0.01). Western blot analysis using specific anti-IL-8 antibody demonstrated the presence of two major immunoreactive bands between 9 and 10 kd, in HPMC culture supernatants. These data demonstrate that HPMC synthesize IL-8 and that its release can be regulated as a result of induction of mRNA expression and de novo protein synthesis by other cytokines.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORNSTEIN M. B. Reconstituted rattail collagen used as substrate for tissue cultures on coverslips in Maximow slides and roller tubes. Lab Invest. 1958 Mar-Apr;7(2):134–137. [PubMed] [Google Scholar]
- Boylan A. M., Rüegg C., Kim K. J., Hébert C. A., Hoeffel J. M., Pytela R., Sheppard D., Goldstein I. M., Broaddus V. C. Evidence of a role for mesothelial cell-derived interleukin 8 in the pathogenesis of asbestos-induced pleurisy in rabbits. J Clin Invest. 1992 Apr;89(4):1257–1267. doi: 10.1172/JCI115710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown Z., Strieter R. M., Chensue S. W., Ceska M., Lindley I., Neild G. H., Kunkel S. L., Westwick J. Cytokine-activated human mesangial cells generate the neutrophil chemoattractant, interleukin 8. Kidney Int. 1991 Jul;40(1):86–90. doi: 10.1038/ki.1991.184. [DOI] [PubMed] [Google Scholar]
- Chung-Welch N., Patton W. F., Yen-Patton G. P., Hechtman H. B., Shepro D. Phenotypic comparison between mesothelial and microvascular endothelial cell lineages using conventional endothelial cell markers, cytoskeletal protein markers and in vitro assays of angiogenic potential. Differentiation. 1989 Oct;42(1):44–53. doi: 10.1111/j.1432-0436.1989.tb00606.x. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Fieren M. W., Van den Bemd G. J., Bonta I. L. Endotoxin-stimulated peritoneal macrophages obtained from continuous ambulatory peritoneal dialysis patients show an increased capacity to release interleukin-1 beta in vitro during infectious peritonitis. Eur J Clin Invest. 1990 Aug;20(4):453–457. doi: 10.1111/j.1365-2362.1990.tb01883.x. [DOI] [PubMed] [Google Scholar]
- Furuta R., Yamagishi J., Kotani H., Sakamoto F., Fukui T., Matsui Y., Sohmura Y., Yamada M., Yoshimura T., Larsen C. G. Production and characterization of recombinant human neutrophil chemotactic factor. J Biochem. 1989 Sep;106(3):436–441. doi: 10.1093/oxfordjournals.jbchem.a122870. [DOI] [PubMed] [Google Scholar]
- Goldman M., Vandenabeele P., Moulart J., Amraoui Z., Abramowicz D., Nortier J., Vanherweghem J. L., Fiers W. Intraperitoneal secretion of interleukin-6 during continuous ambulatory peritoneal dialysis. Nephron. 1990;56(3):277–280. doi: 10.1159/000186154. [DOI] [PubMed] [Google Scholar]
- Goldstein C. S., Bomalaski J. S., Zurier R. B., Neilson E. G., Douglas S. D. Analysis of peritoneal macrophages in continuous ambulatory peritoneal dialysis patients. Kidney Int. 1984 Nov;26(5):733–740. doi: 10.1038/ki.1984.209. [DOI] [PubMed] [Google Scholar]
- Goodman R. B., Wood R. G., Martin T. R., Hanson-Painton O., Kinasewitz G. T. Cytokine-stimulated human mesothelial cells produce chemotactic activity for neutrophils including NAP-1/IL-8. J Immunol. 1992 Jan 15;148(2):457–465. [PubMed] [Google Scholar]
- Gregory H., Young J., Schröder J. M., Mrowietz U., Christophers E. Structure determination of a human lymphocyte derived neutrophil activating peptide (LYNAP). Biochem Biophys Res Commun. 1988 Mar 15;151(2):883–890. doi: 10.1016/s0006-291x(88)80364-4. [DOI] [PubMed] [Google Scholar]
- Hechtman D. H., Cybulsky M. I., Fuchs H. J., Baker J. B., Gimbrone M. A., Jr Intravascular IL-8. Inhibitor of polymorphonuclear leukocyte accumulation at sites of acute inflammation. J Immunol. 1991 Aug 1;147(3):883–892. [PubMed] [Google Scholar]
- Hechtman D. H., Cybulsky M. I., Fuchs H. J., Baker J. B., Gimbrone M. A., Jr Intravascular IL-8. Inhibitor of polymorphonuclear leukocyte accumulation at sites of acute inflammation. J Immunol. 1991 Aug 1;147(3):883–892. [PubMed] [Google Scholar]
- Huber A. R., Kunkel S. L., Todd R. F., 3rd, Weiss S. J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991 Oct 4;254(5028):99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- Hébert C. A., Luscinskas F. W., Kiely J. M., Luis E. A., Darbonne W. C., Bennett G. L., Liu C. C., Obin M. S., Gimbrone M. A., Jr, Baker J. B. Endothelial and leukocyte forms of IL-8. Conversion by thrombin and interactions with neutrophils. J Immunol. 1990 Nov 1;145(9):3033–3040. [PubMed] [Google Scholar]
- Jättelä M. Biologic activities and mechanisms of action of tumor necrosis factor-alpha/cachectin. Lab Invest. 1991 Jun;64(6):724–742. [PubMed] [Google Scholar]
- Kusner D. J., Luebbers E. L., Nowinski R. J., Konieczkowski M., King C. H., Sedor J. R. Cytokine- and LPS-induced synthesis of interleukin-8 from human mesangial cells. Kidney Int. 1991 Jun;39(6):1240–1248. doi: 10.1038/ki.1991.157. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Oppenheim J. J., Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989 Sep;68(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Lindley I., Aschauer H., Seifert J. M., Lam C., Brunowsky W., Kownatzki E., Thelen M., Peveri P., Dewald B., von Tscharner V. Synthesis and expression in Escherichia coli of the gene encoding monocyte-derived neutrophil-activating factor: biological equivalence between natural and recombinant neutrophil-activating factor. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9199–9203. doi: 10.1073/pnas.85.23.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie R. K., Coles G. A., Williams J. D. Eicosanoid synthesis in human peritoneal macrophages stimulated with S. epidermidis. Kidney Int. 1990 May;37(5):1316–1324. doi: 10.1038/ki.1990.117. [DOI] [PubMed] [Google Scholar]
- Mackenzie R. K., Coles G. A., Williams J. D. The response of human peritoneal macrophages to stimulation with bacteria isolated from episodes of continuous ambulatory peritoneal dialysis-related peritonitis. J Infect Dis. 1991 Apr;163(4):837–842. doi: 10.1093/infdis/163.4.837. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Oppenheim J. J. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989 Nov;1(1):2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- Movat H. Z., Burrowes C. E., Cybulsky M. I., Dinarello C. A. Acute inflammation and a Shwartzman-like reaction induced by interleukin-1 and tumor necrosis factor. Synergistic action of the cytokines in the induction of inflammation and microvascular injury. Am J Pathol. 1987 Dec;129(3):463–476. [PMC free article] [PubMed] [Google Scholar]
- Mukaida N., Shiroo M., Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989 Aug 15;143(4):1366–1371. [PubMed] [Google Scholar]
- O'Bryan J. P., Frye R. A., Cogswell P. C., Neubauer A., Kitch B., Prokop C., Espinosa R., 3rd, Le Beau M. M., Earp H. S., Liu E. T. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991 Oct;11(10):5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pötzsch B., Grulich-Henn J., Rössing R., Wille D., Müller-Berghaus G. Identification of endothelial and mesothelial cells in human omental tissue and in omentum-derived cultured cells by specific cell markers. Lab Invest. 1990 Dec;63(6):841–852. [PubMed] [Google Scholar]
- Rankin J. A., Sylvester I., Smith S., Yoshimura T., Leonard E. J. Macrophages cultured in vitro release leukotriene B4 and neutrophil attractant/activation protein (interleukin 8) sequentially in response to stimulation with lipopolysaccharide and zymosan. J Clin Invest. 1990 Nov;86(5):1556–1564. doi: 10.1172/JCI114875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot A. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today. 1992 Aug;13(8):291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Botelho L. H. Endothelins. FASEB J. 1991 Sep;5(12):2713–2720. doi: 10.1096/fasebj.5.12.1916094. [DOI] [PubMed] [Google Scholar]
- Sayers T. J., Wiltrout T. A., Bull C. A., Denn A. C., 3rd, Pilaro A. M., Lokesh B. Effect of cytokines on polymorphonuclear neutrophil infiltration in the mouse. Prostaglandin- and leukotriene-independent induction of infiltration by IL-1 and tumor necrosis factor. J Immunol. 1988 Sep 1;141(5):1670–1677. [PubMed] [Google Scholar]
- Strieter R. M., Phan S. H., Showell H. J., Remick D. G., Lynch J. P., Genord M., Raiford C., Eskandari M., Marks R. M., Kunkel S. L. Monokine-induced neutrophil chemotactic factor gene expression in human fibroblasts. J Biol Chem. 1989 Jun 25;264(18):10621–10626. [PubMed] [Google Scholar]
- Stylianou E., Jenner L. A., Davies M., Coles G. A., Williams J. D. Isolation, culture and characterization of human peritoneal mesothelial cells. Kidney Int. 1990 Jun;37(6):1563–1570. doi: 10.1038/ki.1990.150. [DOI] [PubMed] [Google Scholar]
- Topley N., Floege J., Wessel K., Hass R., Radeke H. H., Kaever V., Resch K. Prostaglandin E2 production is synergistically increased in cultured human glomerular mesangial cells by combinations of IL-1 and tumor necrosis factor-alpha 1. J Immunol. 1989 Sep 15;143(6):1989–1995. [PubMed] [Google Scholar]
- Topley N., Jörres A., Luttmann W., Petersen M. M., Lang M. J., Thierauch K. H., Müller C., Coles G. A., Davies M., Williams J. D. Human peritoneal mesothelial cells synthesize interleukin-6: induction by IL-1 beta and TNF alpha. Kidney Int. 1993 Jan;43(1):226–233. doi: 10.1038/ki.1993.36. [DOI] [PubMed] [Google Scholar]
- Visser M. J., van Bockel J. H., van Muijen G. N., van Hinsbergh V. W. Cells derived from omental fat tissue and used for seeding vascular prostheses are not endothelial in origin. A study on the origin of epitheloid cells derived from omentum. J Vasc Surg. 1991 Mar;13(3):373–381. doi: 10.1067/mva.1991.24480. [DOI] [PubMed] [Google Scholar]
- Wankowicz Z., Megyeri P., Issekutz A. Synergy between tumour necrosis factor alpha and interleukin-1 in the induction of polymorphonuclear leukocyte migration during inflammation. J Leukoc Biol. 1988 Apr;43(4):349–356. doi: 10.1002/jlb.43.4.349. [DOI] [PubMed] [Google Scholar]