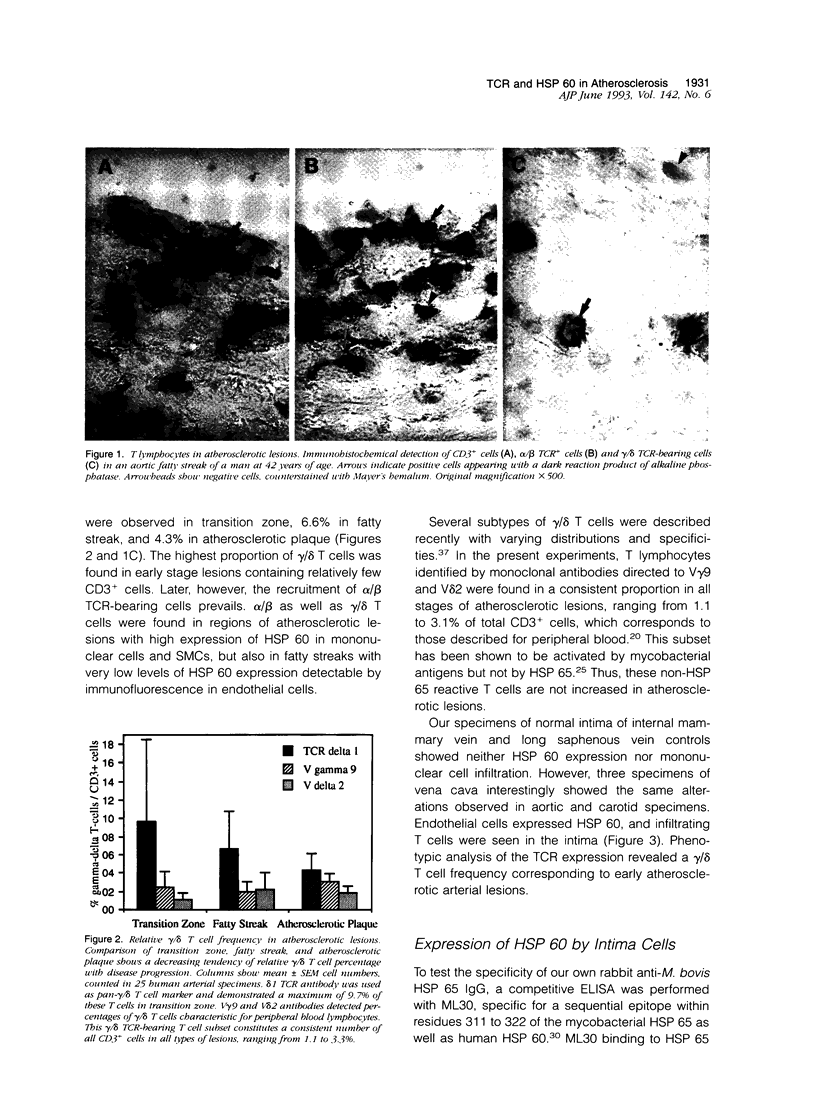
Abstract
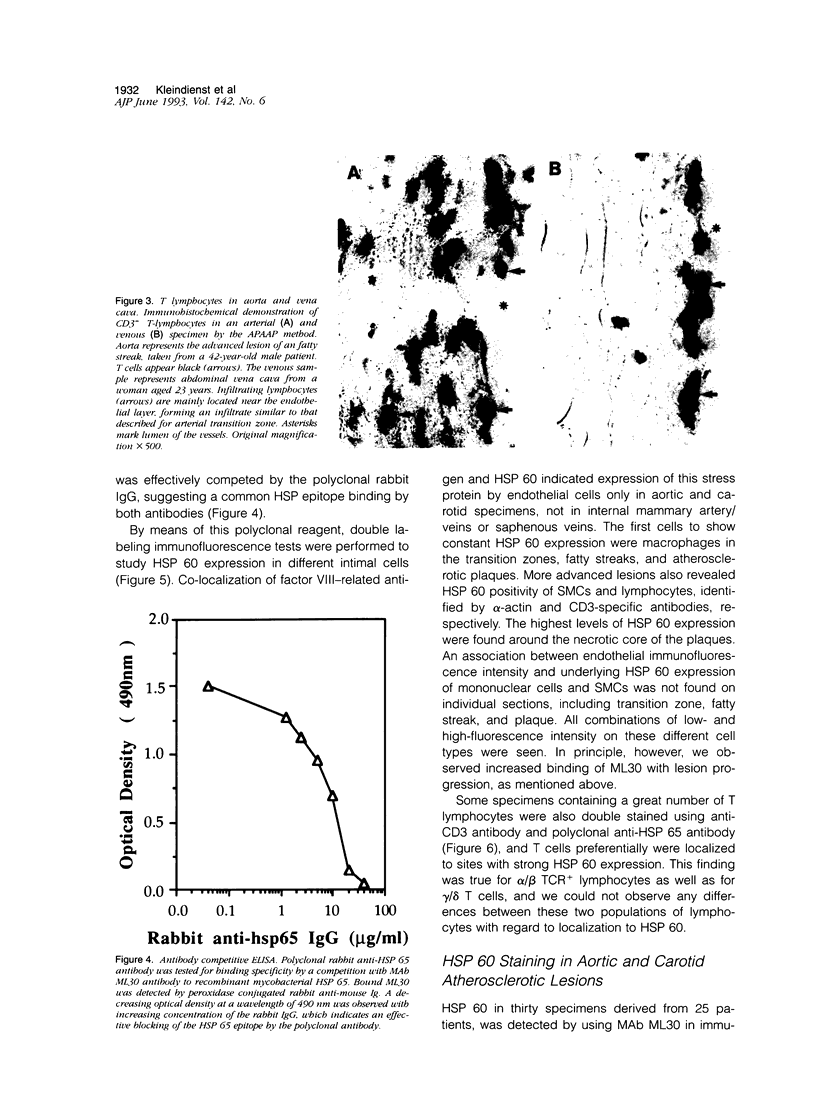
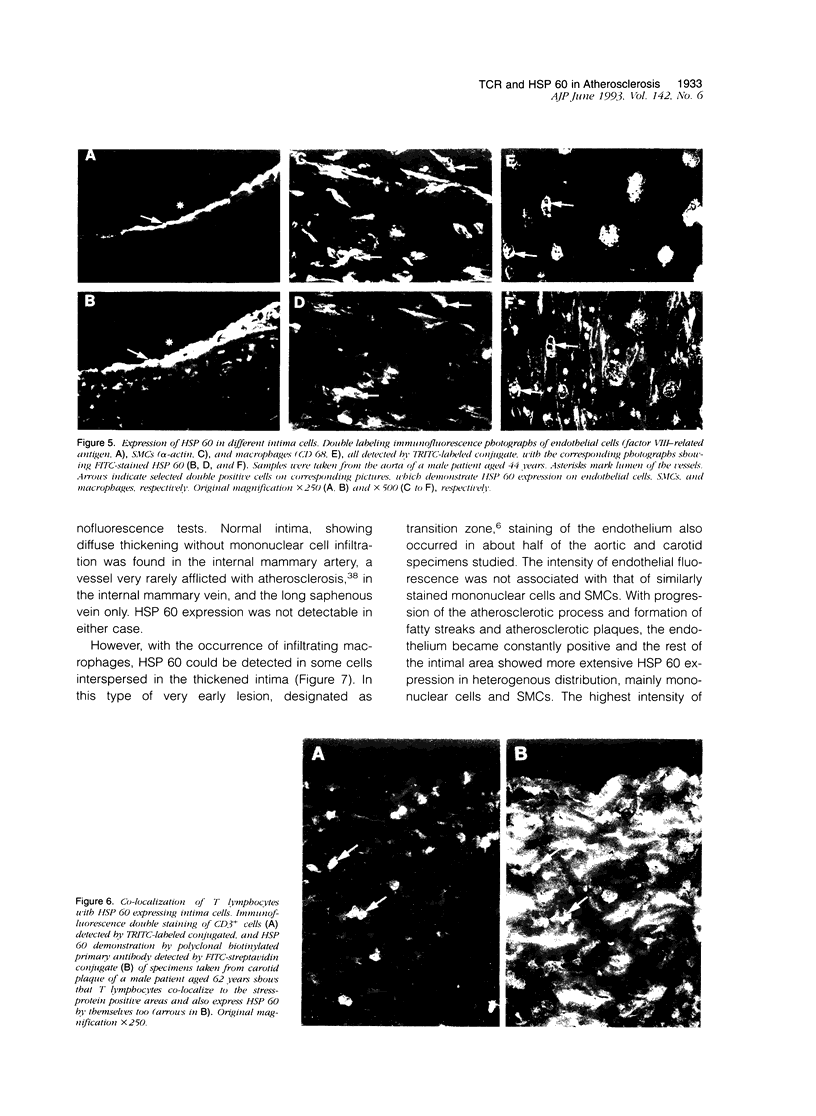
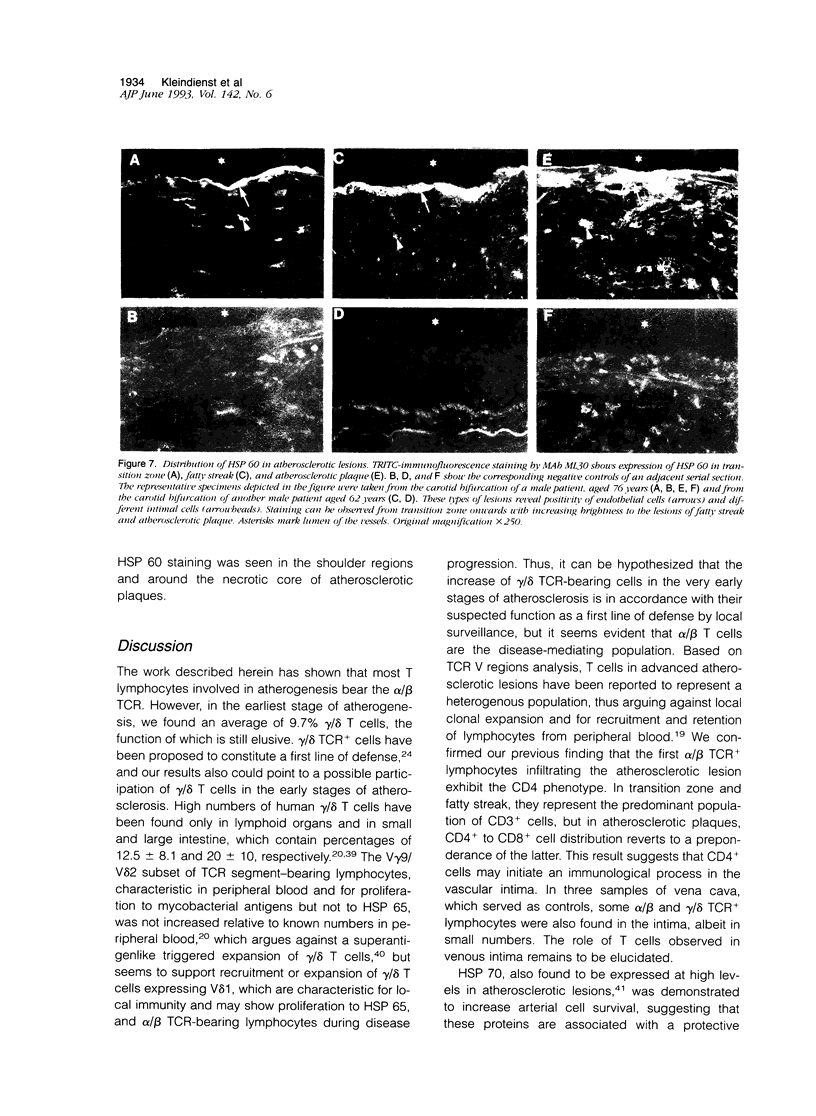
Our previous work revealed the presence of a great number of activated T lymphocytes in early human atherosclerotic lesions, and we were able to induce atherosclerosis in normocholesterolemic rabbits by immunization with Mycobacterium tuberculosis heat-shock protein (HSP) 65. We hypothesized this latter phenomenon to arise from cross-reactivity of mycobacterial HSP 65 with the endogenously expressed homologous 60-kd form of this stress protein. To study HSP 60 expression and the phenotype of intima infiltrating T lymphocytes relative to the T cell receptor (TCR) in human atherosclerotic lesions, specimens of aorta, carotid arteries, and internal mammary arteries and veins, as well as saphenous veins and vena cava from 27 subjects, aged 23 to 80 years, were examined using immunohistochemical and immunofluorescence techniques on serial frozen tissue sections. HSP 60 was detected on endothelium, smooth muscle cells, and/or mononuclear cells of all carotid and aortic specimens, whereas vessels of smaller diameter, serving as reference specimens for normal intima without atherosclerotic lesions and mononuclear infiltration, showed no detectable expression of this stress protein. Furthermore, although the majority of CD3+ cells within the mononuclear cell infiltrates of atherosclerotic lesions bear the alpha/beta TCR, a considerable portion also consisted of gamma/delta TCR+ cells. Thus, 9.7% of T cells in the transition zone between normal intima and fatty streaks carry the gamma/delta TCR, a proportion that decreases to 6.6% and 4.3% in fatty streaks and atherosclerotic plaques, respectively. We conclude that the intensity of HSP 60 expression correlates positively with the atherosclerotic severity and that most lymphocytes participating in atherogenesis bear the alpha/beta TCR, although gamma/delta TCR+ cells are also enriched in atherosclerotic lesions. Expression of HSP 60 by intimal cells, caused, eg, by hemodynamic shear forces, may be responsible for recruitment of HSP-sensitized T cells, thus leading to the induction of an initiating inflammatory process in atherosclerosis. Other risk factors, such as high serum cholesterol levels, contribute to the final outcome of the disease.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr G. M., Rook G. A., al-Saffar M., Van Embden J., Stanford J. L., Behbehani K. Antibody levels to mycobacteria in relation to HLA type: evidence for non-HLA-linked high levels of antibody to the 65 kD heat shock protein of M. bovis in rheumatoid arthritis. Clin Exp Immunol. 1988 Nov;74(2):211–215. [PMC free article] [PubMed] [Google Scholar]
- Berberian P. A., Myers W., Tytell M., Challa V., Bond M. G. Immunohistochemical localization of heat shock protein-70 in normal-appearing and atherosclerotic specimens of human arteries. Am J Pathol. 1990 Jan;136(1):71–80. [PMC free article] [PubMed] [Google Scholar]
- Bucy R. P., Chen C. L., Cooper M. D. Tissue localization and CD8 accessory molecule expression of T gamma delta cells in humans. J Immunol. 1989 May 1;142(9):3045–3049. [PubMed] [Google Scholar]
- Clerget M., Polla B. S. Erythrophagocytosis induces heat shock protein synthesis by human monocytes-macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1081–1085. doi: 10.1073/pnas.87.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornhill J. F., Herderick E. E., Stary H. C. Topography of human aortic sudanophilic lesions. Monogr Atheroscler. 1990;15:13–19. [PubMed] [Google Scholar]
- Elias D., Markovits D., Reshef T., van der Zee R., Cohen I. R. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1576–1580. doi: 10.1073/pnas.87.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeson E. E., Robertson A. L., Jr T lymphocytes in aortic and coronary intimas. Their potential role in atherogenesis. Am J Pathol. 1988 Feb;130(2):369–376. [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Norton P., Ivanyi J. Distribution in tissue sections of the human groEL stress-protein homologue. APMIS. 1990 May;98(5):437–441. doi: 10.1111/j.1699-0463.1990.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Haas W., Kaufman S., Martinez C. The development and function of gamma delta T cells. Immunol Today. 1990 Oct;11(10):340–343. doi: 10.1016/0167-5699(90)90133-t. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989 Jul;135(1):169–175. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Holm J., Claesson-Welsh L. Class II MHC antigen expression in the atherosclerotic plaque: smooth muscle cells express HLA-DR, HLA-DQ and the invariant gamma chain. Clin Exp Immunol. 1986 May;64(2):261–268. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Seifert P. S., Stemme S. Immune mechanisms in atherosclerosis. Arteriosclerosis. 1989 Sep-Oct;9(5):567–578. doi: 10.1161/01.atv.9.5.567. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Seifert P. S., Olsson G., Bondjers G. Immunohistochemical detection of macrophages and T lymphocytes in atherosclerotic lesions of cholesterol-fed rabbits. Arterioscler Thromb. 1991 May-Jun;11(3):745–750. doi: 10.1161/01.atv.11.3.745. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Herendeen S. L., VanBogelen R. A., Neidhardt F. C. Levels of major proteins of Escherichia coli during growth at different temperatures. J Bacteriol. 1979 Jul;139(1):185–194. doi: 10.1128/jb.139.1.185-194.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghirami G., Zhu B. Y., Chess L., Knowles D. M. Flow cytometric and immunohistochemical characterization of the gamma/delta T-lymphocyte population in normal human lymphoid tissue and peripheral blood. Am J Pathol. 1990 Feb;136(2):357–367. [PMC free article] [PubMed] [Google Scholar]
- Johnson A. D., Berberian P. A., Bond M. G. Effect of heat shock proteins on survival of isolated aortic cells from normal and atherosclerotic cynomolgus macaques. Atherosclerosis. 1990 Oct;84(2-3):111–119. doi: 10.1016/0021-9150(90)90080-3. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Gabbiani G., Hansson G. K. Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J Clin Invest. 1985 Jul;76(1):125–131. doi: 10.1172/JCI111934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Heat shock proteins and the immune response. Immunol Today. 1990 Apr;11(4):129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Väth U., Thole J. E., Van Embden J. D., Emmrich F. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur J Immunol. 1987 Mar;17(3):351–357. doi: 10.1002/eji.1830170308. [DOI] [PubMed] [Google Scholar]
- Koga T., Wand-Württenberger A., DeBruyn J., Munk M. E., Schoel B., Kaufmann S. H. T cells against a bacterial heat shock protein recognize stressed macrophages. Science. 1989 Sep 8;245(4922):1112–1115. doi: 10.1126/science.2788923. [DOI] [PubMed] [Google Scholar]
- Libby P., Hansson G. K. Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab Invest. 1991 Jan;64(1):5–15. [PubMed] [Google Scholar]
- Libby P., Salomon R. N., Payne D. D., Schoen F. J., Pober J. S. Functions of vascular wall cells related to development of transplantation-associated coronary arteriosclerosis. Transplant Proc. 1989 Aug;21(4):3677–3684. [PubMed] [Google Scholar]
- Miller A. J., DeBoer A., Palmer A. The role of the lymphatic system in coronary atherosclerosis. Med Hypotheses. 1992 Jan;37(1):31–36. doi: 10.1016/0306-9877(92)90009-2. [DOI] [PubMed] [Google Scholar]
- Minota S., Cameron B., Welch W. J., Winfield J. B. Autoantibodies to the constitutive 73-kD member of the hsp70 family of heat shock proteins in systemic lupus erythematosus. J Exp Med. 1988 Oct 1;168(4):1475–1480. doi: 10.1084/jem.168.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Munro J. M., van der Walt J. D., Munro C. S., Chalmers J. A., Cox E. L. An immunohistochemical analysis of human aortic fatty streaks. Hum Pathol. 1987 Apr;18(4):375–380. doi: 10.1016/s0046-8177(87)80168-5. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Olive C., Gatenby P. A., Serjeantson S. W. Variable gene usage of T cell receptor gamma- and delta-chain transcripts expressed in synovia and peripheral blood of patients with rheumatoid arthritis. Clin Exp Immunol. 1992 Feb;87(2):172–177. doi: 10.1111/j.1365-2249.1992.tb02970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen K. S., Boberg K. M., Stabursvik A., Prydz H. Toxicity of oxygenated cholesterol derivatives toward cultured human umbilical vein endothelial cells. Arterioscler Thromb. 1991 Mar-Apr;11(2):423–428. doi: 10.1161/01.atv.11.2.423. [DOI] [PubMed] [Google Scholar]
- Pfeffer K., Schoel B., Plesnila N., Lipford G. B., Kromer S., Deusch K., Wagner H. A lectin-binding, protease-resistant mycobacterial ligand specifically activates V gamma 9+ human gamma delta T cells. J Immunol. 1992 Jan 15;148(2):575–583. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Seifert P. S., Hansson G. K. Complement receptors and regulatory proteins in human atherosclerotic lesions. Arteriosclerosis. 1989 Nov-Dec;9(6):802–811. doi: 10.1161/01.atv.9.6.802. [DOI] [PubMed] [Google Scholar]
- Seifert P. S., Hugo F., Hansson G. K., Bhakdi S. Prelesional complement activation in experimental atherosclerosis. Terminal C5b-9 complement deposition coincides with cholesterol accumulation in the aortic intima of hypercholesterolemic rabbits. Lab Invest. 1989 Jun;60(6):747–754. [PubMed] [Google Scholar]
- Shinnick T. M. Heat shock proteins as antigens of bacterial and parasitic pathogens. Curr Top Microbiol Immunol. 1991;167:145–160. doi: 10.1007/978-3-642-75875-1_9. [DOI] [PubMed] [Google Scholar]
- Stemme S., Fager G., Hansson G. K. MHC class II antigen expression in human vascular smooth muscle cells is induced by interferon-gamma and modulated by tumour necrosis factor and lymphotoxin. Immunology. 1990 Feb;69(2):243–249. [PMC free article] [PubMed] [Google Scholar]
- Stemme S., Holm J., Hansson G. K. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler Thromb. 1992 Feb;12(2):206–211. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- Stemme S., Rymo L., Hansson G. K. Polyclonal origin of T lymphocytes in human atherosclerotic plaques. Lab Invest. 1991 Dec;65(6):654–660. [PubMed] [Google Scholar]
- Thompson S. J., Rook G. A., Brealey R. J., Van der Zee R., Elson C. J. Autoimmune reactions to heat-shock proteins in pristane-induced arthritis. Eur J Immunol. 1990 Nov;20(11):2479–2484. doi: 10.1002/eji.1830201118. [DOI] [PubMed] [Google Scholar]
- Udelsman R., Blake M. J., Holbrook N. J. Molecular response to surgical stress: specific and simultaneous heat shock protein induction in the adrenal cortex, aorta, and vena cava. Surgery. 1991 Dec;110(6):1125–1131. [PubMed] [Google Scholar]
- Vlaicu R., Niculescu F., Rus H. G., Cristea A. Immunohistochemical localization of the terminal C5b-9 complement complex in human aortic fibrous plaque. Atherosclerosis. 1985 Nov;57(2-3):163–177. doi: 10.1016/0021-9150(85)90030-9. [DOI] [PubMed] [Google Scholar]
- Vroom T. M., Scholte G., Ossendorp F., Borst J. Tissue distribution of human gamma delta T cells: no evidence for general epithelial tropism. J Clin Pathol. 1991 Dec;44(12):1012–1017. doi: 10.1136/jcp.44.12.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q. B., Oberhuber G., Gruschwitz M., Wick G. Immunology of atherosclerosis: cellular composition and major histocompatibility complex class II antigen expression in aortic intima, fatty streaks, and atherosclerotic plaques in young and aged human specimens. Clin Immunol Immunopathol. 1990 Sep;56(3):344–359. doi: 10.1016/0090-1229(90)90155-j. [DOI] [PubMed] [Google Scholar]
- Xu Q., Dietrich H., Steiner H. J., Gown A. M., Schoel B., Mikuz G., Kaufmann S. H., Wick G. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler Thromb. 1992 Jul;12(7):789–799. doi: 10.1161/01.atv.12.7.789. [DOI] [PubMed] [Google Scholar]
- Xu Q., Willeit J., Marosi M., Kleindienst R., Oberhollenzer F., Kiechl S., Stulnig T., Luef G., Wick G. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet. 1993 Jan 30;341(8840):255–259. doi: 10.1016/0140-6736(93)92613-x. [DOI] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]