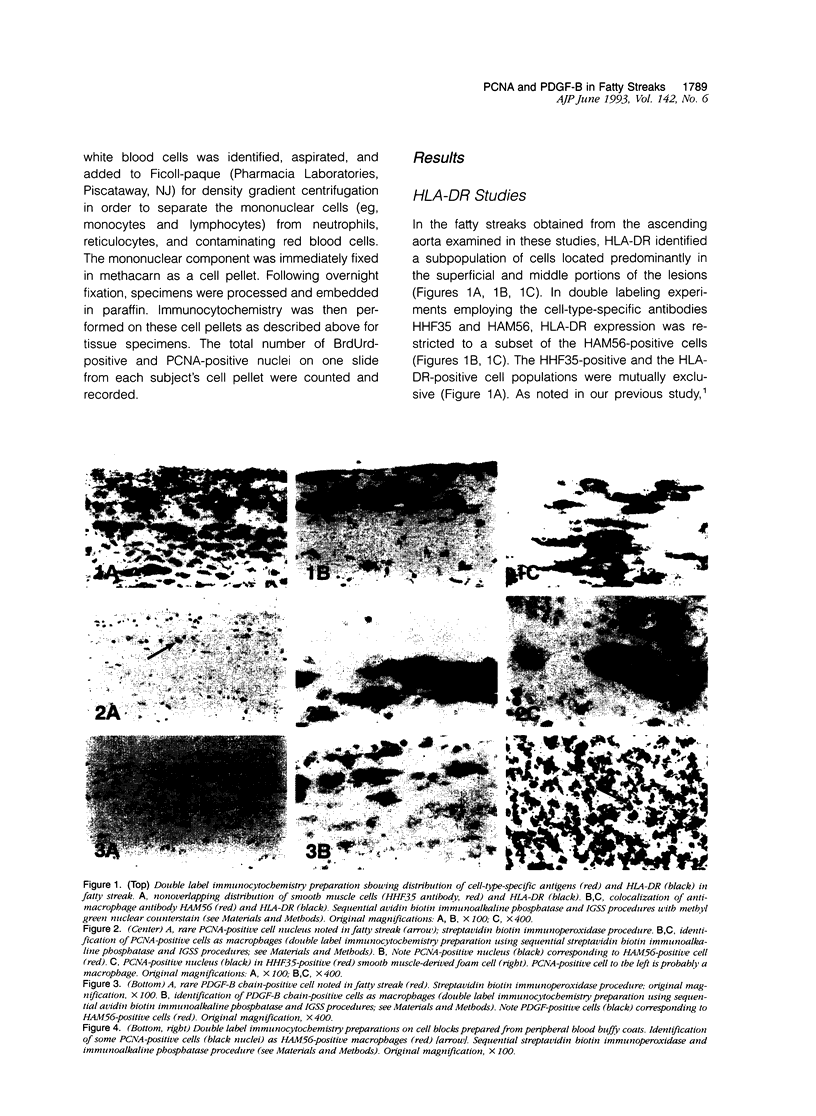
Abstract
The accumulation of smooth muscle cells is a major phenomenon associated with the pathogenesis of lesions of atherosclerosis. Smooth muscle cell proliferation in response to the release of growth factors from neighboring cells, both smooth muscle and macrophages, is one mechanism postulated to account for the increasing numbers of smooth muscle cells as atherosclerotic lesions progress. Indeed, we recently demonstrated the B chain of platelet-derived growth factor (PDGF-B), a potent smooth muscle mitogen, within macrophages in monkey and human lesions of atherosclerosis. To further test the hypothesis that smooth muscle proliferation and/or activation (eg, expression of major histocompatibility complex proteins) plays a role in the early development of these lesions, we applied antibodies to PDGF-B, HLA-DR (a marker of cell activation), and proliferating-associated marker) on a series of early human atherosclerotic lesions from young adults in conjunction with cell-type-specific antibodies. Smooth muscle cells had previously been demonstrated to comprise a major fraction of the cell population in these lesions. In a continuing study of early and intermediate lesions of individuals ranging in age from 15 to 34 years, PDGF-B was detected within macrophages in 2 of 15 lesions. There was no evidence of HLA-DR expression by the smooth muscle cell population in any of the lesions. PCNA-positive cells comprised less than 2% of the cells in the lesions, and the majority of these were blood-borne cells (macrophages and/or lymphocytes), although a small fraction of the PCNA-positive cells were identified as smooth muscle. Concurrent PCNA and 5'-bromodeoxyuridine studies of peripheral blood monocytes demonstrated the presence of significant numbers of cells positive for these proliferation-related markers. It is concluded that the growth factor PDGF-B may have a role in regulating cell proliferation in early human fatty streaks, but the number of proliferating smooth muscle cells is relatively small, and there is no evidence of smooth muscle cell activation, as judged by HLA-DR positivity, in these lesions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelini A., Thiene G., Frescura C., Baroldi G. Coronary arterial wall and atherosclerosis in youth (1-20 years): a histologic study in a northern Italian population. Int J Cardiol. 1990 Sep;28(3):361–370. doi: 10.1016/0167-5273(90)90320-5. [DOI] [PubMed] [Google Scholar]
- Aqel N. M., Ball R. Y., Waldmann H., Mitchinson M. J. Identification of macrophages and smooth muscle cells in human atherosclerosis using monoclonal antibodies. J Pathol. 1985 Jul;146(3):197–204. doi: 10.1002/path.1711460306. [DOI] [PubMed] [Google Scholar]
- Coltrera M. D., Gown A. M. PCNA/cyclin expression and BrdU uptake define different subpopulations in different cell lines. J Histochem Cytochem. 1991 Jan;39(1):23–30. doi: 10.1177/39.1.1670579. [DOI] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Garcia R. L., Coltrera M. D., Gown A. M. Analysis of proliferative grade using anti-PCNA/cyclin monoclonal antibodies in fixed, embedded tissues. Comparison with flow cytometric analysis. Am J Pathol. 1989 Apr;134(4):733–739. [PMC free article] [PubMed] [Google Scholar]
- Giordano M., Danova M., Pellicciari C., Wilson G. D., Mazzini G., Conti A. M., Franchini G., Riccardi A., Romanini M. G. Proliferating cell nuclear antigen (PCNA)/cyclin expression during the cell cycle in normal and leukemic cells. Leuk Res. 1991;15(11):965–974. doi: 10.1016/0145-2126(91)90101-x. [DOI] [PubMed] [Google Scholar]
- Gordon D., Reidy M. A., Benditt E. P., Schwartz S. M. Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4600–4604. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Holm J., Claesson-Welsh L. Class II MHC antigen expression in the atherosclerotic plaque: smooth muscle cells express HLA-DR, HLA-DQ and the invariant gamma chain. Clin Exp Immunol. 1986 May;64(2):261–268. [PMC free article] [PubMed] [Google Scholar]
- Katsuda S., Boyd H. C., Fligner C., Ross R., Gown A. M. Human atherosclerosis. III. Immunocytochemical analysis of the cell composition of lesions of young adults. Am J Pathol. 1992 Apr;140(4):907–914. [PMC free article] [PubMed] [Google Scholar]
- Klurfeld D. M. Identification of foam cells in human atherosclerotic lesions as macrophages using monoclonal antibodies. Arch Pathol Lab Med. 1985 May;109(5):445–449. [PubMed] [Google Scholar]
- Kurki P., Lotz M., Ogata K., Tan E. M. Proliferating cell nuclear antigen (PCNA)/cyclin in activated human T lymphocytes. J Immunol. 1987 Jun 15;138(12):4114–4120. [PubMed] [Google Scholar]
- Kurki P., Ogata K., Tan E. M. Monoclonal antibodies to proliferating cell nuclear antigen (PCNA)/cyclin as probes for proliferating cells by immunofluorescence microscopy and flow cytometry. J Immunol Methods. 1988 Apr 22;109(1):49–59. doi: 10.1016/0022-1759(88)90441-3. [DOI] [PubMed] [Google Scholar]
- Munro J. M., van der Walt J. D., Munro C. S., Chalmers J. A., Cox E. L. An immunohistochemical analysis of human aortic fatty streaks. Hum Pathol. 1987 Apr;18(4):375–380. doi: 10.1016/s0046-8177(87)80168-5. [DOI] [PubMed] [Google Scholar]
- Orekhov A. N., Andreeva E. R., Krushinsky A. V., Novikov I. D., Tertov V. V., Nestaiko G. V., Khashimov Kh A., Repin V. S., Smirnov V. N. Intimal cells and atherosclerosis. Relationship between the number of intimal cells and major manifestations of atherosclerosis in the human aorta. Am J Pathol. 1986 Nov;125(2):402–415. [PMC free article] [PubMed] [Google Scholar]
- Roessner A., Herrera A., Höning H. J., Vollmer E., Zwadlo G., Schürmann R., Sorg C., Grundmann E. Identification of macrophages and smooth muscle cells with monoclonal antibodies in the human atherosclerotic plaque. Virchows Arch A Pathol Anat Histopathol. 1987;412(2):169–174. doi: 10.1007/BF00716190. [DOI] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Stemme S., Fager G., Hansson G. K. MHC class II antigen expression in human vascular smooth muscle cells is induced by interferon-gamma and modulated by tumour necrosis factor and lymphotoxin. Immunology. 1990 Feb;69(2):243–249. [PMC free article] [PubMed] [Google Scholar]
- Thaete L. G., Ahnen D. J., Malkinson A. M. Proliferating cell nuclear antigen (PCNA/cyclin) immunocytochemistry as a labeling index in mouse lung tissues. Cell Tissue Res. 1989 Apr;256(1):167–173. doi: 10.1007/BF00224731. [DOI] [PubMed] [Google Scholar]
- Vedeler C. A., Nyland H., Matre R. In situ characterization of the foam cells in early human atherosclerotic lesions. Acta Pathol Microbiol Immunol Scand C. 1984 Apr;92(2):133–137. doi: 10.1111/j.1699-0463.1984.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Velican C., Velican D. Study of coronary intimal thickening. Atherosclerosis. 1985 Sep;56(3):331–344. doi: 10.1016/0021-9150(85)90008-5. [DOI] [PubMed] [Google Scholar]
- Warner S. J., Friedman G. B., Libby P. Regulation of major histocompatibility gene expression in human vascular smooth muscle cells. Arteriosclerosis. 1989 May-Jun;9(3):279–288. doi: 10.1161/01.atv.9.3.279. [DOI] [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Williams L. T., Schwartz S. M., Gordon D. Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J Clin Invest. 1988 Sep;82(3):1134–1143. doi: 10.1172/JCI113671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissler R. W., Vesselinovitch D., Komatsu A. The contribution of studies of atherosclerotic lesions in young people to future research. Ann N Y Acad Sci. 1990;598:418–434. doi: 10.1111/j.1749-6632.1990.tb42313.x. [DOI] [PubMed] [Google Scholar]
- Xu Q. B., Oberhuber G., Gruschwitz M., Wick G. Immunology of atherosclerosis: cellular composition and major histocompatibility complex class II antigen expression in aortic intima, fatty streaks, and atherosclerotic plaques in young and aged human specimens. Clin Immunol Immunopathol. 1990 Sep;56(3):344–359. doi: 10.1016/0090-1229(90)90155-j. [DOI] [PubMed] [Google Scholar]
- Yoshino T., Mukuzono H., Aoki H., Takahashi K., Takeuchi T., Kubonishi I., Ohtsuki Y., Motoi M., Akagi T. A novel monoclonal antibody (OPD4) recognizing a helper/inducer T cell subset. Its application to paraffin-embedded tissues. Am J Pathol. 1989 Jun;134(6):1339–1346. [PMC free article] [PubMed] [Google Scholar]






