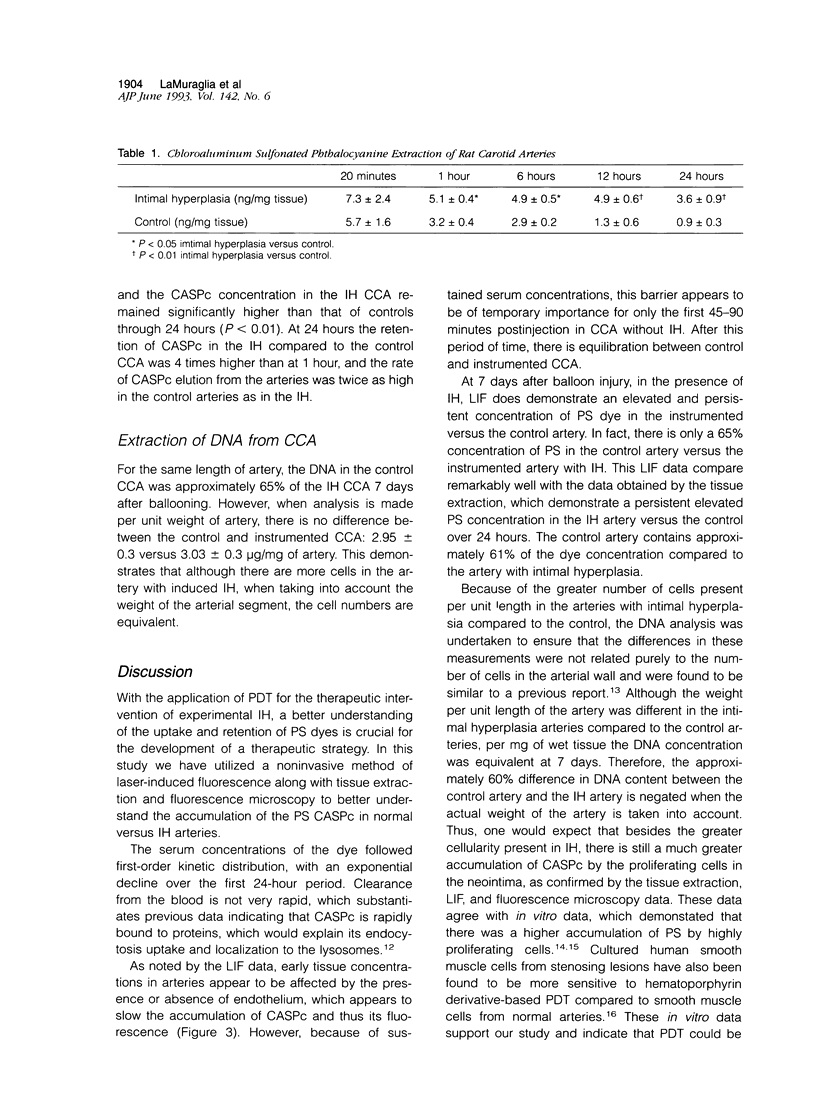
Abstract
Photodynamic therapy, the light activation of photosensitizers into cytotoxic mediators, has been a successful treatment for experimental intimal hyperplasia (IH). To understand the basis of the photosensitizer chloroaluminum sulfonated phthalocyanine (CASPc)-mediated photoinhibition of intimal hyperplasia in the rat common carotid artery model, we studied photosensitizer partitioning in hyperplastic as compared to normal arterial tissue. Serum clearance of CASPc is exponential with, a half-life of 300 minutes. Laser-induced fluorescence and spectrofluorimetric analyses of artery tissue demonstrated an approximately 60% lower uptake and retention of CASPc by normal arterial tissue as compared to arteries with IH; the differences become more pronounced at 24 h. Fluorescent microscopy of arterial tissue demonstrated increased uptake of the CASPc by the artery with IH. However, by 24 h it is primarily the IH tissue that has retained the CASPc, with clearance of the dye from the media of normal or hyperplastic arteries. These data demonstrate that IH, like neoplastic tissue, has an increased accumulation of CASPc compared to normal artery. The preferential partitioning into hyperplastic tissue has implications for therapeutic targeting of this cellular population with photodynamic therapy.
Full text
PDF
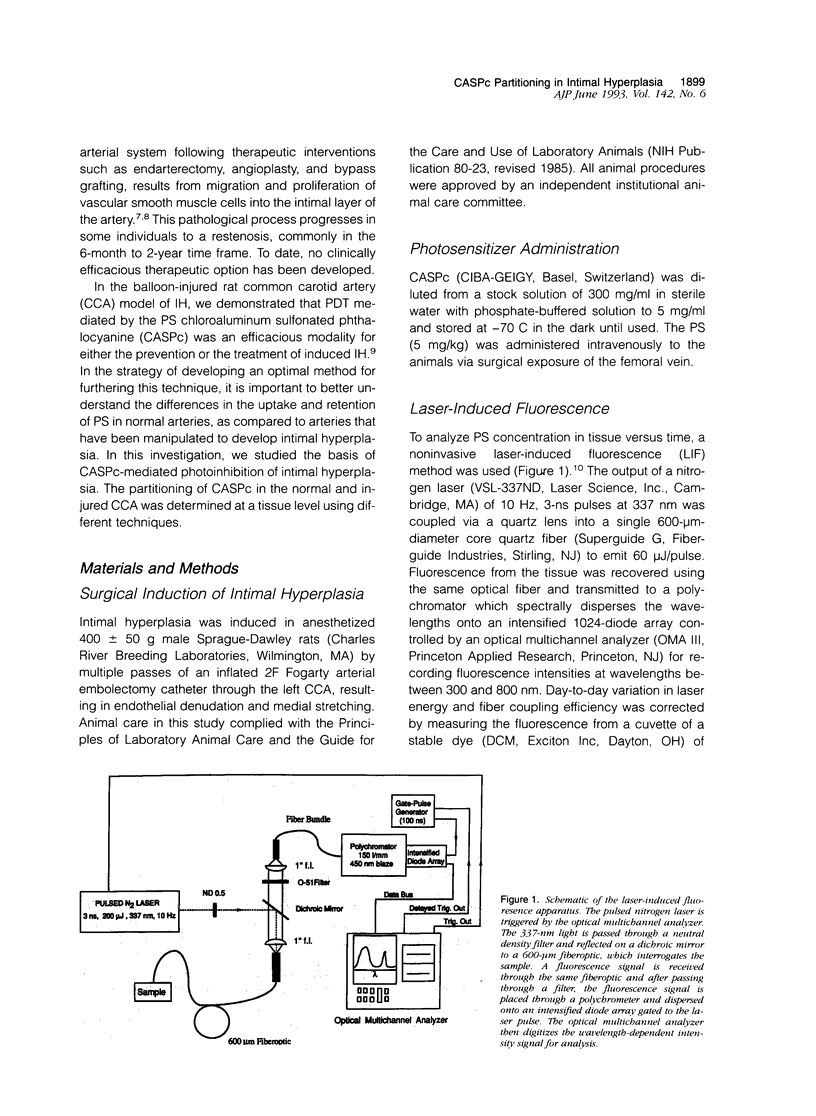

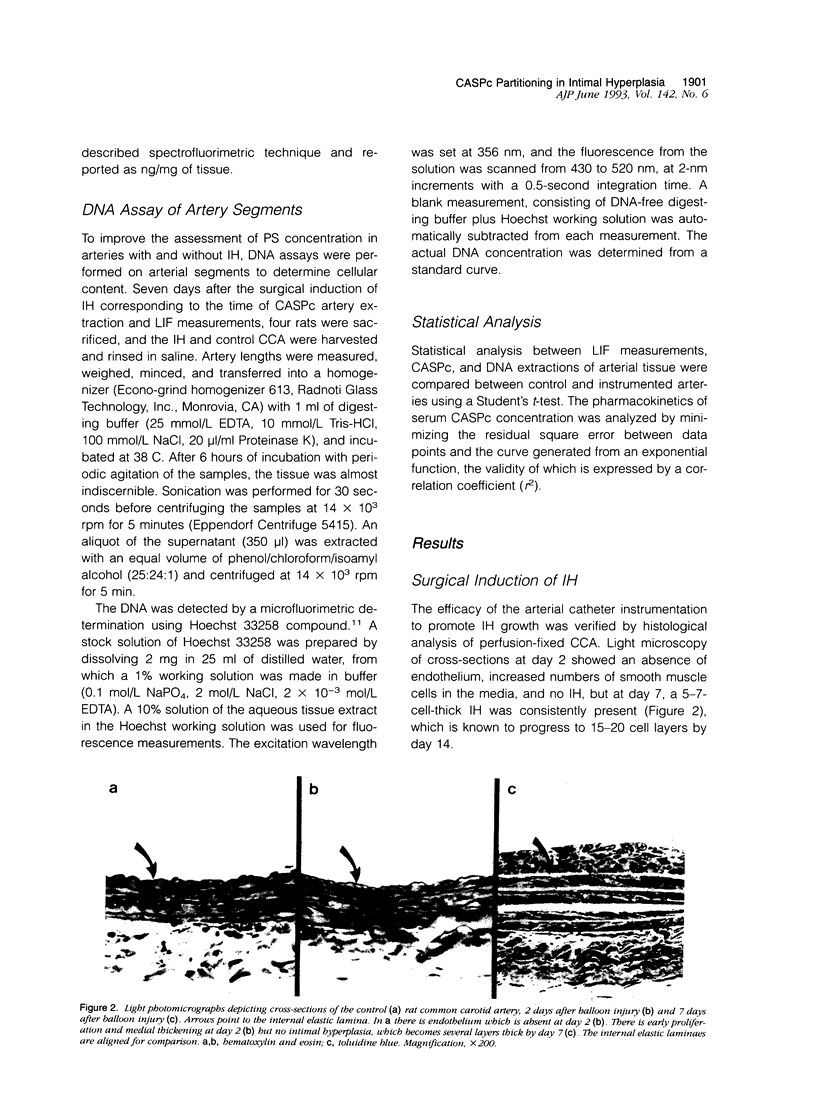
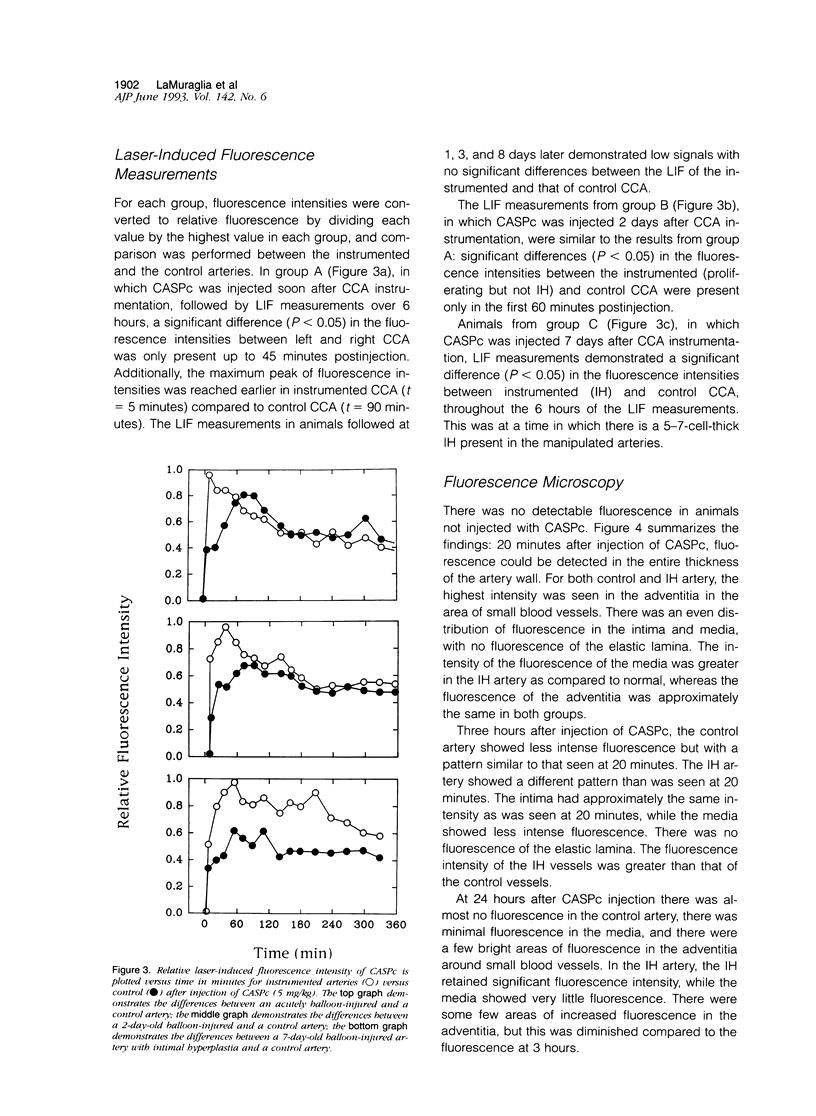
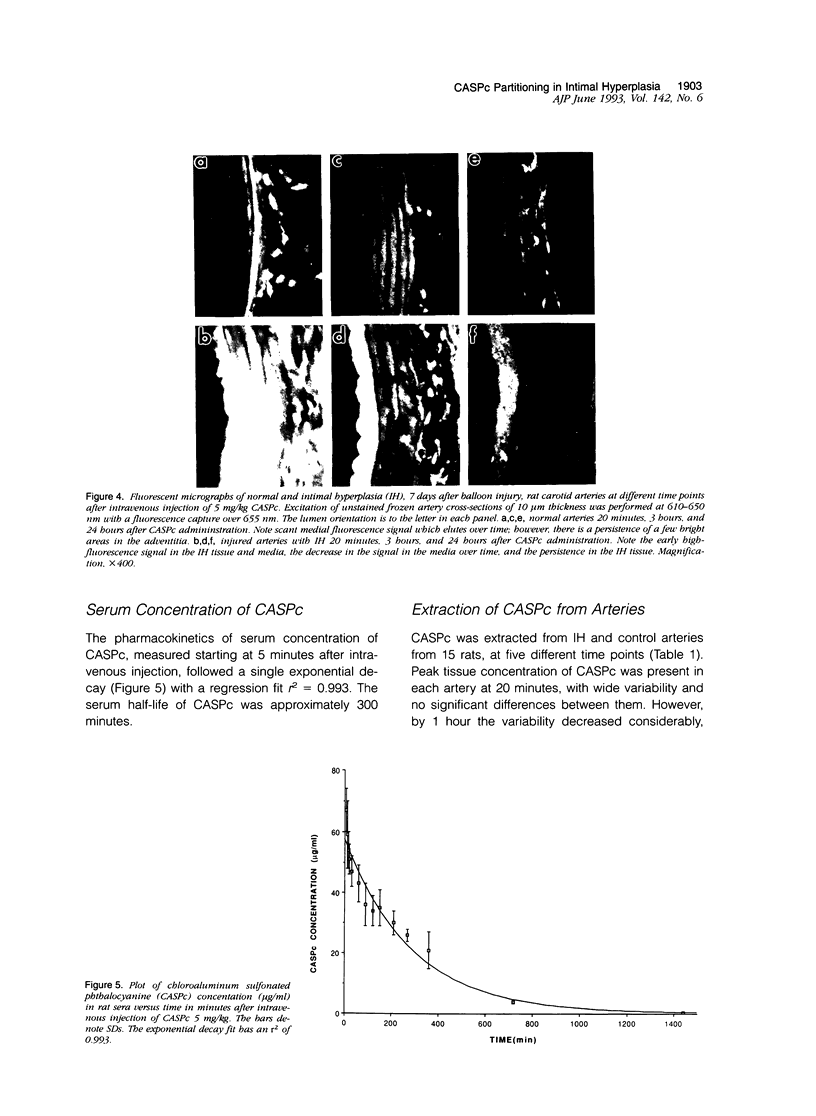

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cesarone C. F., Bolognesi C., Santi L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem. 1979 Nov 15;100(1):188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Mechanisms of stenosis after arterial injury. Lab Invest. 1983 Aug;49(2):208–215. [PubMed] [Google Scholar]
- Dartsch P. C., Ischinger T., Betz E. Responses of cultured smooth muscle cells from human nonatherosclerotic arteries and primary stenosing lesions after photoradiation: implications for photodynamic therapy of vascular stenoses. J Am Coll Cardiol. 1990 Jun;15(7):1545–1550. doi: 10.1016/0735-1097(90)92824-l. [DOI] [PubMed] [Google Scholar]
- Gomer C. J., Rucker N., Murphree A. L. Differential cell photosensitivity following porphyrin photodynamic therapy. Cancer Res. 1988 Aug 15;48(16):4539–4542. [PubMed] [Google Scholar]
- Ip J. H., Fuster V., Badimon L., Badimon J., Taubman M. B., Chesebro J. H. Syndromes of accelerated atherosclerosis: role of vascular injury and smooth muscle cell proliferation. J Am Coll Cardiol. 1990 Jun;15(7):1667–1687. doi: 10.1016/0735-1097(90)92845-s. [DOI] [PubMed] [Google Scholar]
- Kocher O., Gabbiani F., Gabbiani G., Reidy M. A., Cokay M. S., Peters H., Hüttner I. Phenotypic features of smooth muscle cells during the evolution of experimental carotid artery intimal thickening. Biochemical and morphologic studies. Lab Invest. 1991 Oct;65(4):459–470. [PubMed] [Google Scholar]
- Moan J., Steen H. B., Feren K., Christensen T. Uptake of hematoporphyrin derivative and sensitized photoinactivation of C3H cells with different oncogenic potential. Cancer Lett. 1981 Dec;14(3):291–296. doi: 10.1016/0304-3835(81)90157-9. [DOI] [PubMed] [Google Scholar]
- Mosley S. T., Goldstein J. L., Brown M. S., Falck J. R., Anderson R. G. Targeted killing of cultured cells by receptor-dependent photosensitization. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5717–5721. doi: 10.1073/pnas.78.9.5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortu P., LaMuraglia G. M., Roberts W. G., Flotte T. J., Hasan T. Photodynamic therapy of arteries. A novel approach for treatment of experimental intimal hyperplasia. Circulation. 1992 Mar;85(3):1189–1196. doi: 10.1161/01.cir.85.3.1189. [DOI] [PubMed] [Google Scholar]
- Roberts W. G., Berns M. W. In vitro photosensitization I. Cellular uptake and subcellular localization of mono-L-aspartyl chlorin e6, chloro-aluminum sulfonated phthalocyanine, and photofrin II. Lasers Surg Med. 1989;9(2):90–101. doi: 10.1002/lsm.1900090203. [DOI] [PubMed] [Google Scholar]
- Roberts W. G., Hasan T. Role of neovasculature and vascular permeability on the tumor retention of photodynamic agents. Cancer Res. 1992 Feb 15;52(4):924–930. [PubMed] [Google Scholar]
- Roberts W. G., Klein M. K., Loomis M., Weldy S., Berns M. W. Photodynamic therapy of spontaneous cancers in felines, canines, and snakes with chloro-aluminum sulfonated phthalocyanine. J Natl Cancer Inst. 1991 Jan 2;83(1):18–23. doi: 10.1093/jnci/83.1.18. [DOI] [PubMed] [Google Scholar]
- Rosenthal I. Phthalocyanines as photodynamic sensitizers. Photochem Photobiol. 1991 Jun;53(6):859–870. doi: 10.1111/j.1751-1097.1991.tb09900.x. [DOI] [PubMed] [Google Scholar]
- West C. M., West D. C., Kumar S., Moore J. V. A comparison of the sensitivity to photodynamic treatment of endothelial and tumour cells in different proliferative states. Int J Radiat Biol. 1990 Jul;58(1):145–156. doi: 10.1080/09553009014551501. [DOI] [PubMed] [Google Scholar]




