Abstract
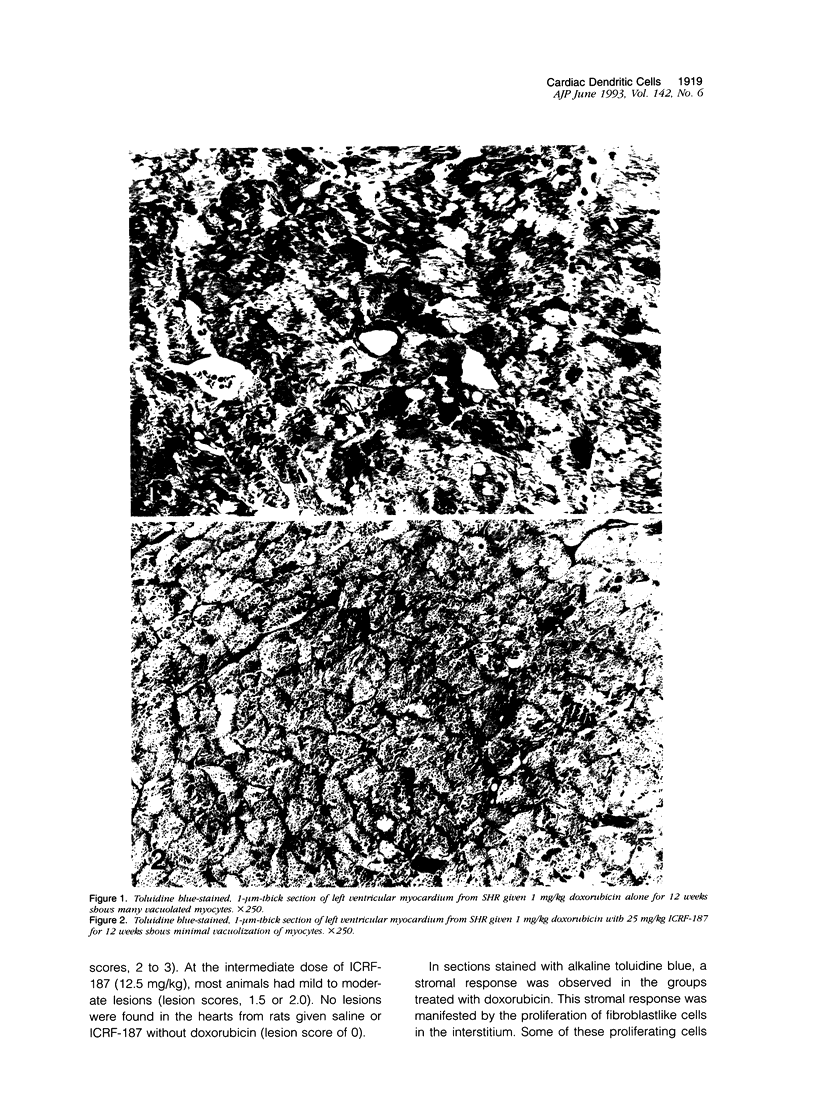
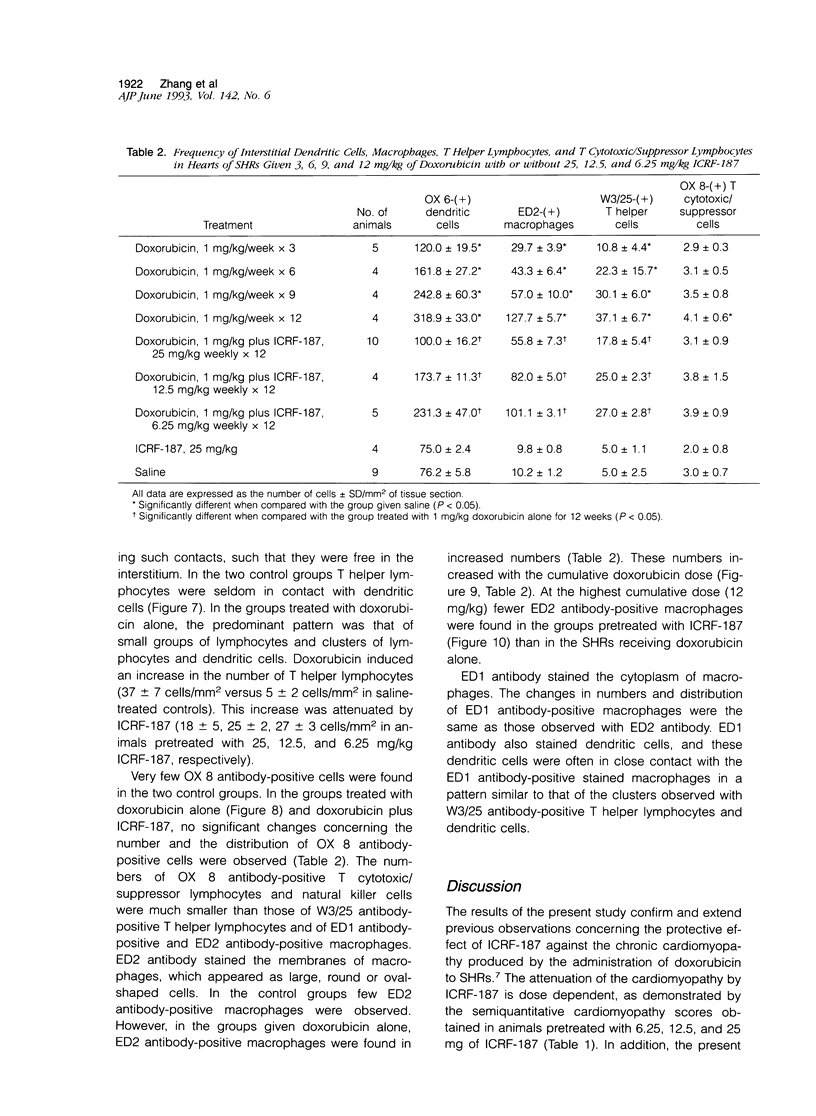
Histological and immunohistochemical studies using specific monoclonal antibodies were made to evaluate the severity of the chronic cardiomyopathy and the quantitative changes in interstitial dendritic cells (antigen-presenting cells), T helper lymphocytes, T cytotoxic/suppressor lymphocytes, and macrophages in the hearts of spontaneously hypertensive rats (SHRs) treated with doxorubicin at 1 mg/kg per week for 3, 6, 9 or 12 weeks. In addition, an assessment was made of the modifications of the responses of these cell populations by pretreatment of the SHR with ICRF-187, which protects against doxorubicin cardiotoxicity. The number of interstitial dendritic cells/mm2 of section of left ventricle was similar in saline-treated control SHRs (76 +/- 6) and in those treated with ICRF-187 alone (75 +/- 2) but increased markedly (319 +/- 33) in animals receiving a total cumulative dose of 12 mg/kg doxorubicin. Treatment with ICRF-187 prior to each administration of doxorubicin attenuated in a dose-dependent manner the increase in numbers of dendritic cells induced by doxorubicin (231 +/- 47, 174 +/- 11, and 100 +/- 16 cells/mm2) after treatment with 6.25, 12.5, and 25 mg of ICRF-187, respectively. Doxorubicin also induced increases in the numbers of T helper lymphocytes and macrophages but not of T cytotoxic/suppressor lymphocytes. These increases were also attenuated by pretreatment with ICRF-187. These data were interpreted as indicating that doxorubicin cardiotoxicity results in the release of substances that initiate immune reactions involving the antigen-presenting cells of the heart and that such reactions are attenuated by pretreatment with ICRF-187.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul Hamied T. A., Turk J. L. Enhancement of interleukin-2 release in rats by treatment with bleomycin and adriamycin in vivo. Cancer Immunol Immunother. 1987;25(3):245–249. doi: 10.1007/BF00199154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arinaga S., Akiyoshi T., Tsuji H. Augmentation of the generation of cell-mediated cytotoxicity after a single dose of adriamycin in cancer patients. Cancer Res. 1986 Aug;46(8):4213–4216. [PubMed] [Google Scholar]
- Balsari A., Alzani R., Parrello D., Morelli D., Tagliabue E., Gianni L., Isetta A. M., Menard S., Colnaghi M. I., Ghione M. Monoclonal antibodies against doxorubicin. Int J Cancer. 1988 Nov 15;42(5):798–802. doi: 10.1002/ijc.2910420528. [DOI] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Cohen S. A., Salazar D., Wicher J. Adriamycin-induced activation of NK activity may initially involve LAF production. Cancer Immunol Immunother. 1983;15(3):188–193. doi: 10.1007/BF00199163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. P., Campigotto B. M., Johnson J. G., Elliott B. E. Differential growth inhibition and enhancement of major histocompatibility complex class I antigen expression by interferons in a small-cell lung cancer cell line and its doxorubicin-selected multidrug-resistant variant. Cancer Immunol Immunother. 1991;33(4):274–277. doi: 10.1007/BF01744948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Ehrke M. J., Maccubbin D., Ryoyama K., Cohen S. A., Mihich E. Correlation between adriamycin-induced augmentation of interleukin 2 production and of cell-mediated cytotoxicity in mice. Cancer Res. 1986 Jan;46(1):54–60. [PubMed] [Google Scholar]
- Ehrke M. J., Ryoyama K., Cohen S. A. Cellular basis for adriamycin-induced augmentation of cell-mediated cytotoxicity in culture. Cancer Res. 1984 Jun;44(6):2497–2504. [PubMed] [Google Scholar]
- Green J., Jotte R. Interactions between T helper cells and dendritic cells during the rat mixed lymphocyte reaction. J Exp Med. 1985 Nov 1;162(5):1546–1560. doi: 10.1084/jem.162.5.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
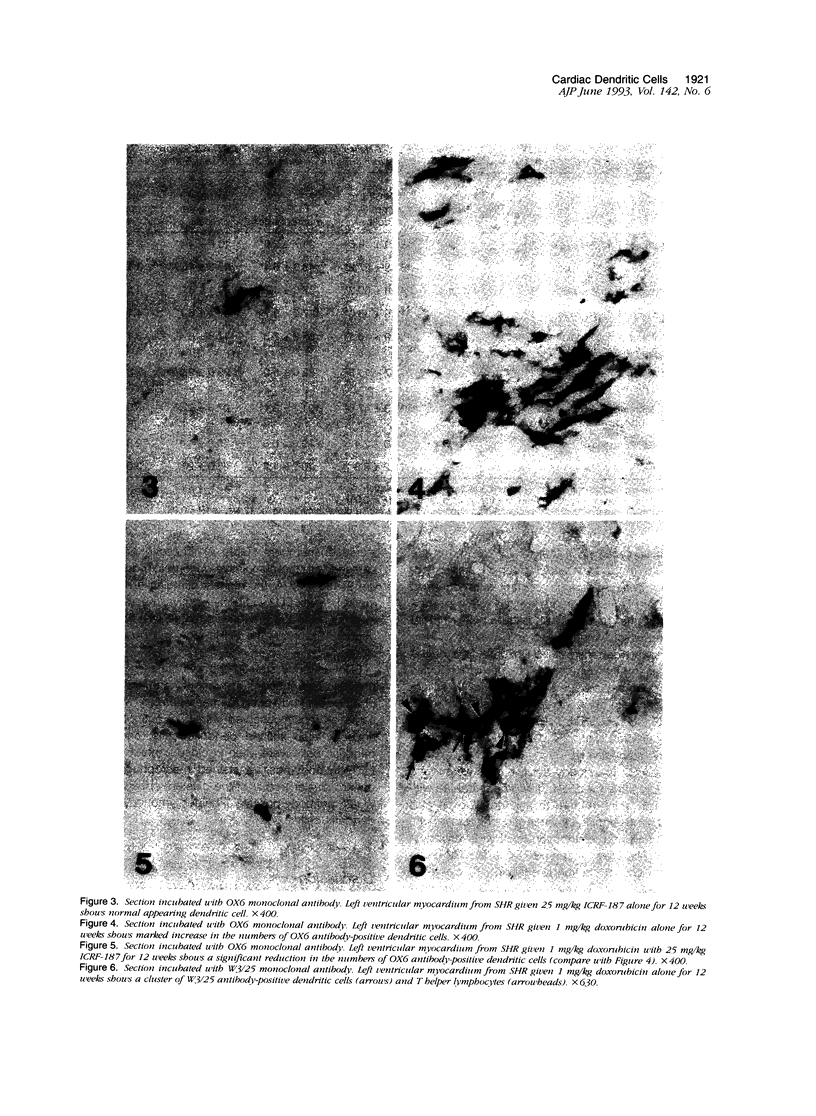
- Hart D. N., Fabre J. W. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J Exp Med. 1981 Aug 1;154(2):347–361. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasinoff B. B. The interaction of the cardioprotective agent ICRF-187 [+)-1,2-bis(3,5-dioxopiperazinyl-1-yL)propane); its hydrolysis product (ICRF-198); and other chelating agents with the Fe(III) and Cu(II) complexes of adriamycin. Agents Actions. 1989 Mar;26(3-4):378–385. doi: 10.1007/BF01967305. [DOI] [PubMed] [Google Scholar]
- Haskill J. S. Adriamycin-activated macrophages as tumor growth inhibitors. Cancer Res. 1981 Oct;41(10):3852–3856. [PubMed] [Google Scholar]
- Herman E. H., Ferrans V. J. Influence of vitamin E and ICRF-187 on chronic doxorubicin cardiotoxicity in miniature swine. Lab Invest. 1983 Jul;49(1):69–77. [PubMed] [Google Scholar]
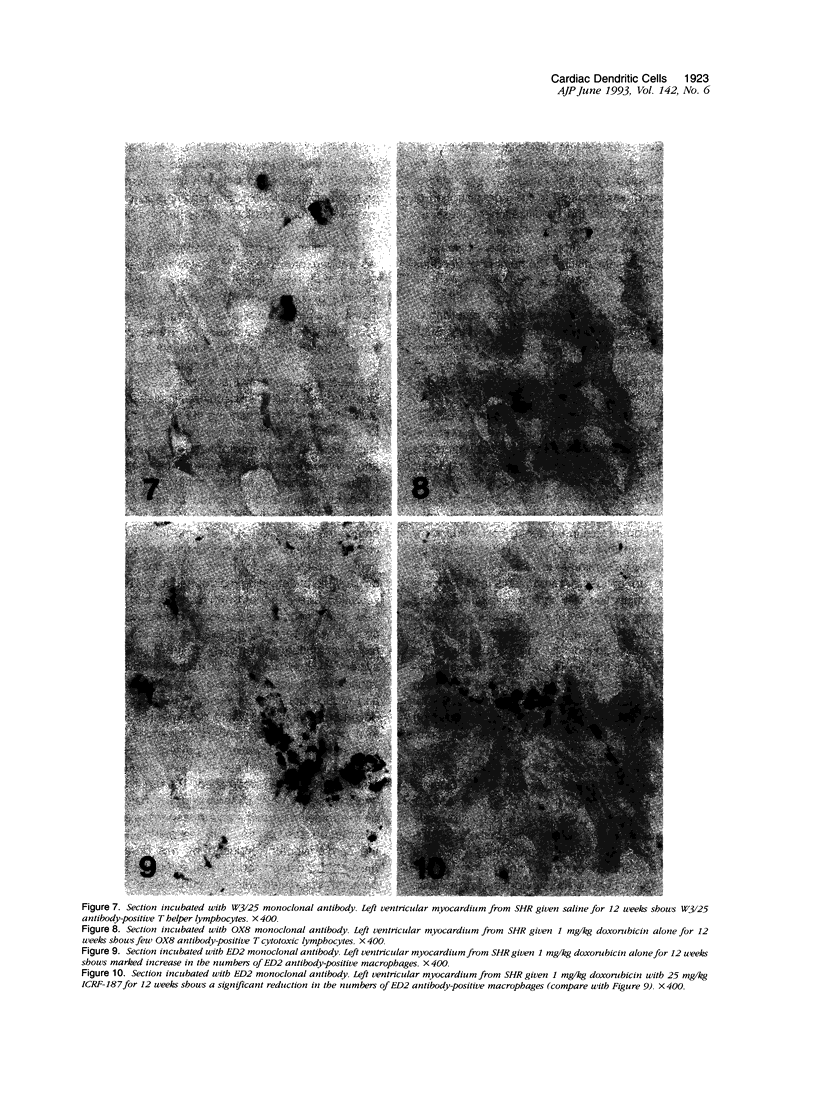
- Herman E. H., Ferrans V. J., Myers C. E., Van Vleet J. F. Comparison of the effectiveness of (+/-)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane (ICRF-187) and N-acetylcysteine in preventing chronic doxorubicin cardiotoxicity in beagles. Cancer Res. 1985 Jan;45(1):276–281. [PubMed] [Google Scholar]
- Herman E. H., Ferrans V. J. Reduction of chronic doxorubicin cardiotoxicity in dogs by pretreatment with (+/-)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane (ICRF-187). Cancer Res. 1981 Sep;41(9 Pt 1):3436–3440. [PubMed] [Google Scholar]
- Herman E. H., Ferrans V. J., Young R. S., Hamlin R. L. Effect of pretreatment with ICRF-187 on the total cumulative dose of doxorubicin tolerated by beagle dogs. Cancer Res. 1988 Dec 1;48(23):6918–6925. [PubMed] [Google Scholar]
- Herman E. H., el-Hage A., Ferrans V. J. Protective effect of ICRF-187 on doxorubicin-induced cardiac and renal toxicity in spontaneously hypertensive (SHR) and normotensive (WKY) rats. Toxicol Appl Pharmacol. 1988 Jan;92(1):42–53. doi: 10.1016/0041-008x(88)90226-8. [DOI] [PubMed] [Google Scholar]
- Huber S. A. Doxorubicin-induced alterations in cultured myocardial cells stimulate cytolytic T lymphocyte responses. Am J Pathol. 1990 Aug;137(2):449–456. [PMC free article] [PubMed] [Google Scholar]
- Maccubbin D. L., Whitman J. A., Taniguchi N., Mace K. F., Ehrke M. J., Mihich E. Comparison of adriamycin induced immunomodulation with that of the noncardiotoxic anthracycline 5-iminodaunorubicin. Int J Immunopharmacol. 1988;10(3):317–323. doi: 10.1016/0192-0561(88)90064-1. [DOI] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Myers C. E., Gianni L., Simone C. B., Klecker R., Greene R. Oxidative destruction of erythrocyte ghost membranes catalyzed by the doxorubicin-iron complex. Biochemistry. 1982 Apr 13;21(8):1707–1712. doi: 10.1021/bi00537a001. [DOI] [PubMed] [Google Scholar]
- Nio Y., Imai S., Shiraishi T., Ohgaki K., Tobe T. Lymphocytes anticancer chemosensitivity testing in vitro--an approach to predict immunosuppressive effect of anticancer agents. J Clin Lab Immunol. 1989 Jul;29(3):141–145. [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Perkins W. E., Schroeder R. L., Carrano R. A., Imondi A. R. Effect of ICRF-187 on doxorubicin-induced myocardial effects in the mouse and guinea pig. Br J Cancer. 1982 Oct;46(4):662–667. doi: 10.1038/bjc.1982.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S. C., Fabre J. W. Characterization of the tissue macrophage and the interstitial dendritic cell as distinct leukocytes normally resident in the connective tissue of rat heart. J Exp Med. 1990 Jun 1;171(6):1841–1851. doi: 10.1084/jem.171.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speyer J. L., Green M. D., Kramer E., Rey M., Sanger J., Ward C., Dubin N., Ferrans V., Stecy P., Zeleniuch-Jacquotte A. Protective effect of the bispiperazinedione ICRF-187 against doxorubicin-induced cardiac toxicity in women with advanced breast cancer. N Engl J Med. 1988 Sep 22;319(12):745–752. doi: 10.1056/NEJM198809223191203. [DOI] [PubMed] [Google Scholar]
- Speyer J. L., Green M. D., Zeleniuch-Jacquotte A., Wernz J. C., Rey M., Sanger J., Kramer E., Ferrans V., Hochster H., Meyers M. ICRF-187 permits longer treatment with doxorubicin in women with breast cancer. J Clin Oncol. 1992 Jan;10(1):117–127. doi: 10.1200/JCO.1992.10.1.117. [DOI] [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J Exp Med. 1982 Dec 1;156(6):1780–1793. doi: 10.1084/jem.156.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiniger B., Klempnauer J., Wonigeit K. Phenotype and histological distribution of interstitial dendritic cells in the rat pancreas, liver, heart, and kidney. Transplantation. 1984 Aug;38(2):169–174. doi: 10.1097/00007890-198408000-00016. [DOI] [PubMed] [Google Scholar]
- Van Schie R. C., De Mulder P. H., Verstraten H. G., Van Rennes H., Wagener D. J. In vitro studies on the influence of doxorubicin in combination with recombinant interferon-gamma on human monocytes. Anticancer Res. 1991 May-Jun;11(3):1245–1252. [PubMed] [Google Scholar]
- White R. A., Mason D. W., Williams A. F., Galfre G., Milstein C. T-lymphocyte heterogeneity in the rat: separation of functional subpopulations using a monoclonal antibody. J Exp Med. 1978 Sep 1;148(3):664–673. doi: 10.1084/jem.148.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]