Abstract
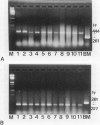
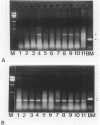
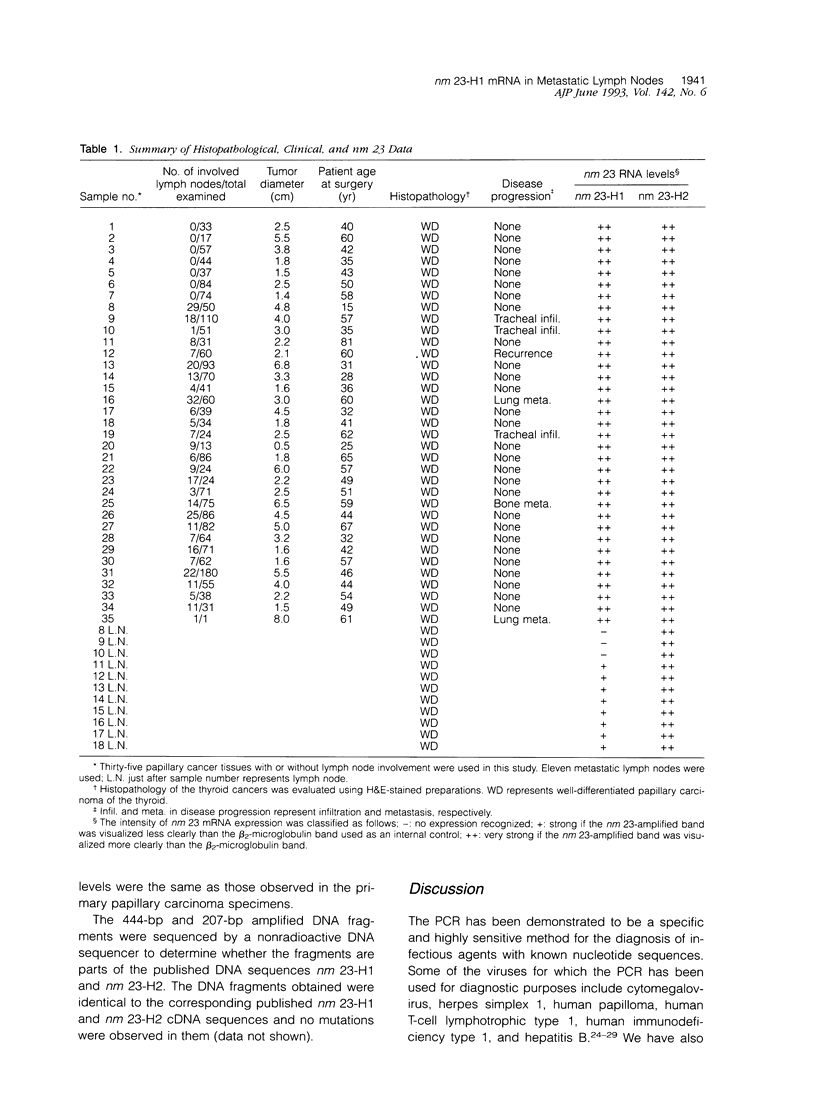
The nm 23 gene product, which possesses nucleoside diphosphate kinase activity, is a possible mediator of cancer cell invasion and metastasis. It has been divided into two distinct gene products, nm23-H1 and nm23-H2. We have developed a method for detecting nm 23-H1 and nm 23-H2 RNA using the polymerase chain reaction, based on the amplification of complementary DNA copies of nm 23-H1 and nm 23-H2 RNA. Using this method, the nm 23-H1 and nm 23-H2 messenger (m)RNA levels in 35 thyroid papillary carcinomas, 11 metastatic lymph nodes from patients with thyroid papillary carcinomas, five thyroid follicular adenomas, and three normal thyroid tissue samples were studied. Both nm 23-H1 and nm 23-H2 mRNA were expressed ubiquitously in normal and malignant thyroid tissues. However, in metastatic lymph nodes no (3 of 11) or weak expression (8 of 11) of nm 23-H1 mRNA was observed, the extent of which was inversely proportional to the degree of cancer cell occupancy, whereas nm 23-H2 mRNA was expressed and the levels were similar to those in other tissue tested. These results show that nm 23-H1 only may play a role in cancer cell invasion and metastasis although the exact mechanisms involved have yet to be elucidated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Ansorge W., Sproat B. S., Stegemann J., Schwager C. A non-radioactive automated method for DNA sequence determination. J Biochem Biophys Methods. 1986 Dec;13(6):315–323. doi: 10.1016/0165-022x(86)90038-2. [DOI] [PubMed] [Google Scholar]
- Bevilacqua G., Sobel M. E., Liotta L. A., Steeg P. S. Association of low nm23 RNA levels in human primary infiltrating ductal breast carcinomas with lymph node involvement and other histopathological indicators of high metastatic potential. Cancer Res. 1989 Sep 15;49(18):5185–5190. [PubMed] [Google Scholar]
- Biggs J., Hersperger E., Steeg P. S., Liotta L. A., Shearn A. A Drosophila gene that is homologous to a mammalian gene associated with tumor metastasis codes for a nucleoside diphosphate kinase. Cell. 1990 Nov 30;63(5):933–940. doi: 10.1016/0092-8674(90)90496-2. [DOI] [PubMed] [Google Scholar]
- Cao M., Xiao X., Egbert B., Darragh T. M., Yen T. S. Rapid detection of cutaneous herpes simplex virus infection with the polymerase chain reaction. J Invest Dermatol. 1989 Mar;92(3):391–392. doi: 10.1111/1523-1747.ep12277232. [DOI] [PubMed] [Google Scholar]
- Chehab F. F., Xiao X., Kan Y. W., Yen T. S. Detection of cytomegalovirus infection in paraffin-embedded tissue specimens with the polymerase chain reaction. Mod Pathol. 1989 Mar;2(2):75–78. [PubMed] [Google Scholar]
- Duggan D. B., Ehrlich G. D., Davey F. P., Kwok S., Sninsky J., Goldberg J., Baltrucki L., Poiesz B. J. HTLV-I-induced lymphoma mimicking Hodgkin's disease. Diagnosis by polymerase chain reaction amplification of specific HTLV-I sequences in tumor DNA. Blood. 1988 Apr;71(4):1027–1032. [PubMed] [Google Scholar]
- Fidler I. J., Balch C. M. The biology of cancer metastasis and implications for therapy. Curr Probl Surg. 1987 Mar;24(3):129–209. doi: 10.1016/0011-3840(87)90002-5. [DOI] [PubMed] [Google Scholar]
- Gilles A. M., Presecan E., Vonica A., Lascu I. Nucleoside diphosphate kinase from human erythrocytes. Structural characterization of the two polypeptide chains responsible for heterogeneity of the hexameric enzyme. J Biol Chem. 1991 May 15;266(14):8784–8789. [PubMed] [Google Scholar]
- Haut M., Steeg P. S., Willson J. K., Markowitz S. D. Induction of nm23 gene expression in human colonic neoplasms and equal expression in colon tumors of high and low metastatic potential. J Natl Cancer Inst. 1991 May 15;83(10):712–716. doi: 10.1093/jnci/83.10.712. [DOI] [PubMed] [Google Scholar]
- Juliano R. L. Membrane receptors for extracellular matrix macromolecules: relationship to cell adhesion and tumor metastasis. Biochim Biophys Acta. 1987 Nov 25;907(3):261–278. doi: 10.1016/0304-419x(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Kimura N., Shimada N., Nomura K., Watanabe K. Isolation and characterization of a cDNA clone encoding rat nucleoside diphosphate kinase. J Biol Chem. 1990 Sep 15;265(26):15744–15749. [PubMed] [Google Scholar]
- Kwok S., Mack D. H., Mullis K. B., Poiesz B., Ehrlich G., Blair D., Friedman-Kien A., Sninsky J. J. Identification of human immunodeficiency virus sequences by using in vitro enzymatic amplification and oligomer cleavage detection. J Virol. 1987 May;61(5):1690–1694. doi: 10.1128/jvi.61.5.1690-1694.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larzul D., Guigue F., Sninsky J. J., Mack D. H., Bréchot C., Guesdon J. L. Detection of hepatitis B virus sequences in serum by using in vitro enzymatic amplification. J Virol Methods. 1988 Jul;20(3):227–237. doi: 10.1016/0166-0934(88)90126-7. [DOI] [PubMed] [Google Scholar]
- Leone A., Flatow U., King C. R., Sandeen M. A., Margulies I. M., Liotta L. A., Steeg P. S. Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm23-transfected melanoma cells. Cell. 1991 Apr 5;65(1):25–35. doi: 10.1016/0092-8674(91)90404-m. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Biochemical mechanisms of tumor invasion and metastases. Clin Physiol Biochem. 1987;5(3-4):190–199. [PubMed] [Google Scholar]
- Nicolson G. L. Cell surface molecules and tumor metastasis. Regulation of metastatic phenotypic diversity. Exp Cell Res. 1984 Jan;150(1):3–22. doi: 10.1016/0014-4827(84)90696-7. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Tumor cell instability, diversification, and progression to the metastatic phenotype: from oncogene to oncofetal expression. Cancer Res. 1987 Mar 15;47(6):1473–1487. [PubMed] [Google Scholar]
- Rosengard A. M., Krutzsch H. C., Shearn A., Biggs J. R., Barker E., Margulies I. M., King C. R., Liotta L. A., Steeg P. S. Reduced Nm23/Awd protein in tumour metastasis and aberrant Drosophila development. Nature. 1989 Nov 9;342(6246):177–180. doi: 10.1038/342177a0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Giancotti F. G. Integrins and tumor cell dissemination. Cancer Cells. 1989 Dec;1(4):119–126. [PubMed] [Google Scholar]
- Sakakura T., Kusano I. Tenascin in tissue perturbation repair. Acta Pathol Jpn. 1991 Apr;41(4):247–258. doi: 10.1111/j.1440-1827.1991.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V. Cancer metastasis: experimental approaches, theoretical concepts, and impacts for treatment strategies. Adv Cancer Res. 1985;43:1–73. doi: 10.1016/s0065-230x(08)60942-2. [DOI] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl J. A., Leone A., Rosengard A. M., Porter L., King C. R., Steeg P. S. Identification of a second human nm23 gene, nm23-H2. Cancer Res. 1991 Jan 1;51(1):445–449. [PubMed] [Google Scholar]
- Steeg P. S., Bevilacqua G., Kopper L., Thorgeirsson U. P., Talmadge J. E., Liotta L. A., Sobel M. E. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst. 1988 Apr 6;80(3):200–204. doi: 10.1093/jnci/80.3.200. [DOI] [PubMed] [Google Scholar]
- Steeg P. S., Bevilacqua G., Pozzatti R., Liotta L. A., Sobel M. E. Altered expression of NM23, a gene associated with low tumor metastatic potential, during adenovirus 2 Ela inhibition of experimental metastasis. Cancer Res. 1988 Nov 15;48(22):6550–6554. [PubMed] [Google Scholar]
- Sugawara I., Watanabe M., Masunaga A., Itoyama S., Ueda K. Primer-dependent amplification of mdr1 mRNA by polymerase chain reaction. Jpn J Cancer Res. 1992 Feb;83(2):131–133. doi: 10.1111/j.1349-7006.1992.tb00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss H., Schwager C., Wirkner U., Sproat B., Zimmermann J., Rosenthal A., Erfle H., Stegemann J., Ansorge W. Direct genomic fluorescent on-line sequencing and analysis using in vitro amplification of DNA. Nucleic Acids Res. 1989 Apr 11;17(7):2517–2527. doi: 10.1093/nar/17.7.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]