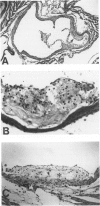
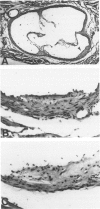
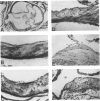
Abstract
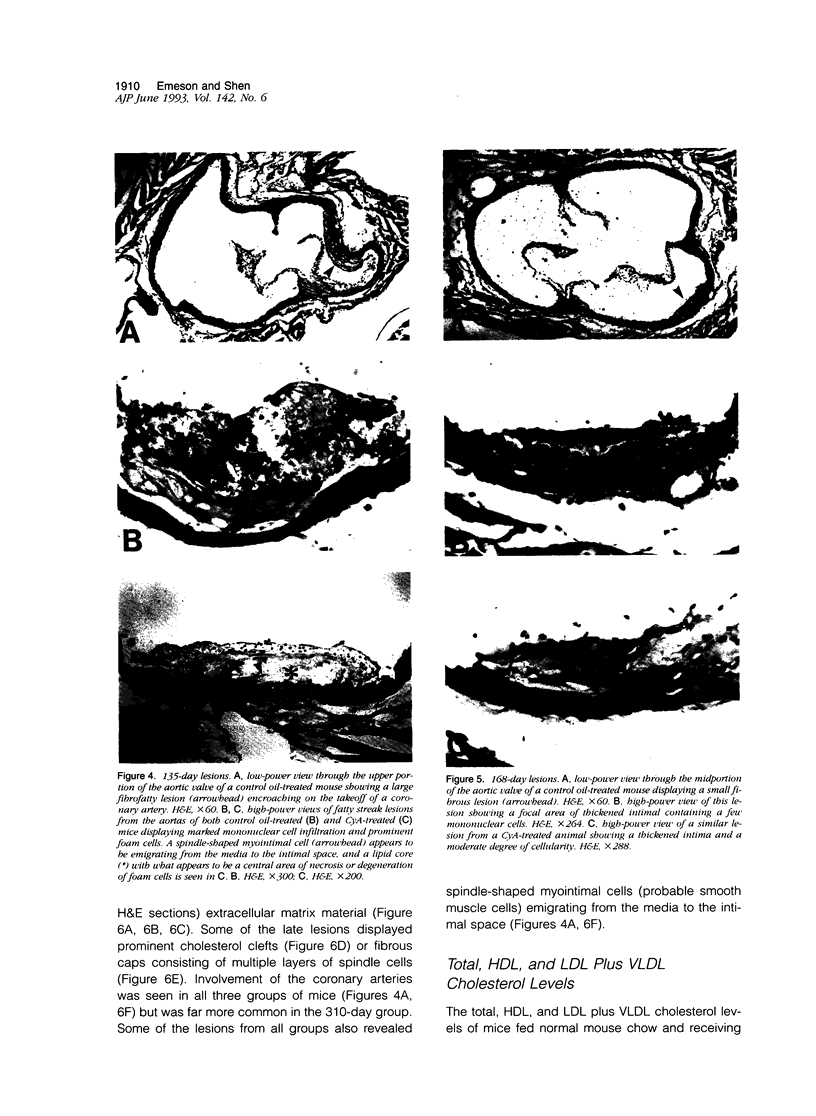
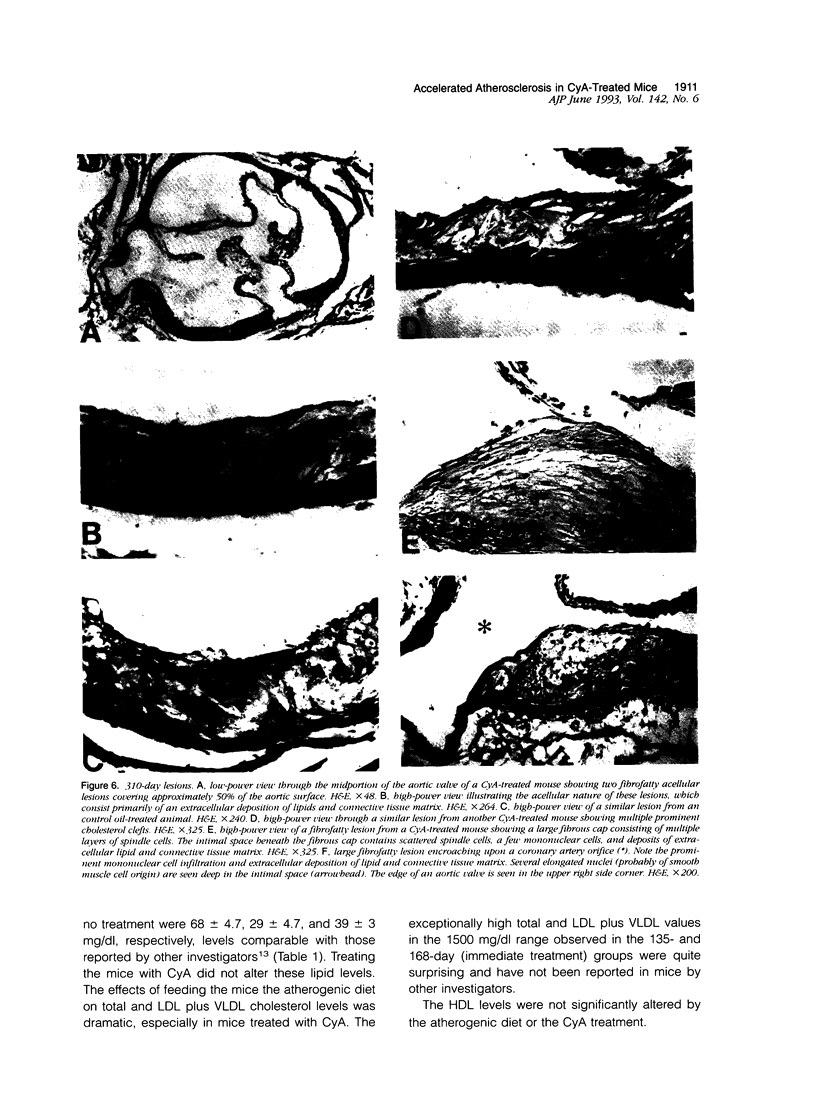
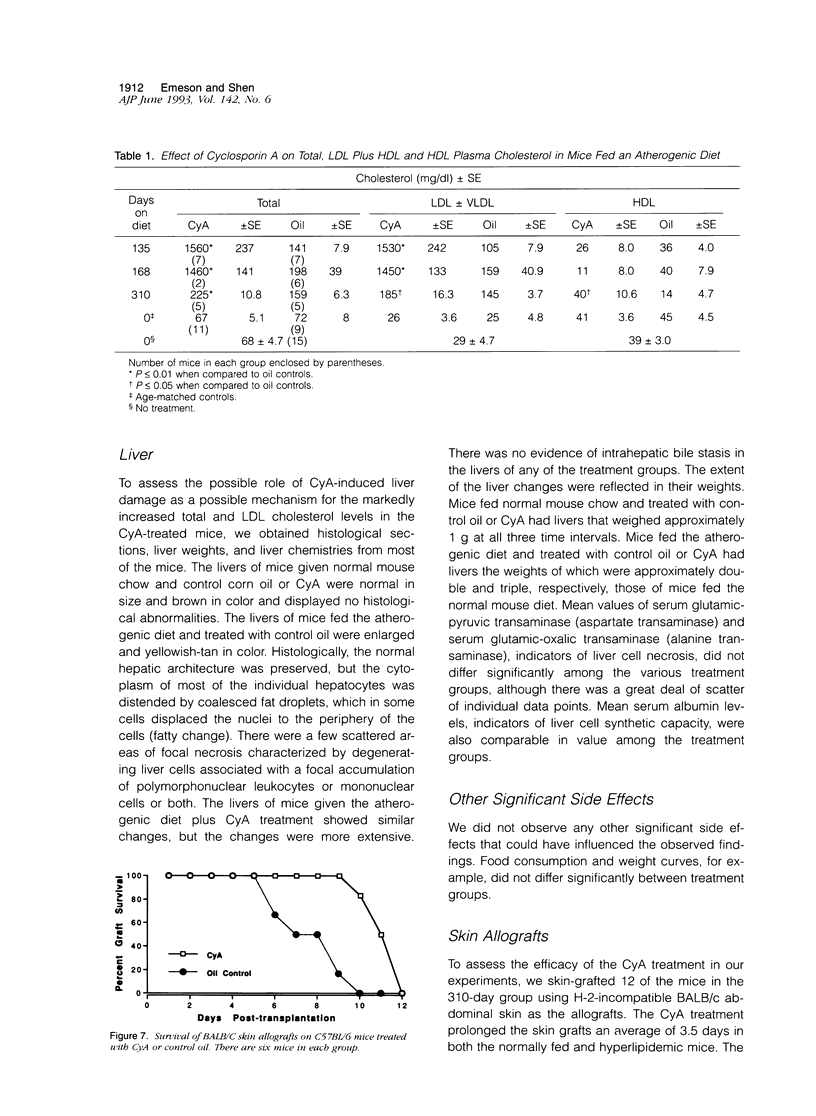
We and others have demonstrated that T lymphocytes are prominent components of atherosclerotic lesions. We hypothesized that if T cells were necessary for the development of atherosclerosis it would be possible to demonstrate its prevention or retardation in T-cell-suppressed mice. To test this hypothesis, CyA, a potent suppressor of T-cell activation, was used to treat C57BL/6 mice undergoing lipid hyperalimentation. Mice receiving normal mouse chow were completely free of atherosclerotic lesions. In mice receiving the atherogenic diet plus control oil injections, lesions of the aorta and coronary arteries were observed at 135 days and increased progressively in area until 310 days. Somewhat surprisingly, mice given the atherogenic diet plus CyA injections displayed even larger lesions at all three observed time intervals. Although CyA did suppress T-cell reactivity sufficiently to obtain the expected prolongation of skin allografts, it did not suppress the development or progression of atherosclerotic lesions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auchincloss H., Jr, Winn H. J. Murine CD8+ T cell helper function is particularly sensitive to cyclosporine suppression in vivo. J Immunol. 1989 Dec 15;143(12):3940–3943. [PubMed] [Google Scholar]
- Ballantyne C. M., Podet E. J., Patsch W. P., Harati Y., Appel V., Gotto A. M., Jr, Young J. B. Effects of cyclosporine therapy on plasma lipoprotein levels. JAMA. 1989 Jul 7;262(1):53–56. [PubMed] [Google Scholar]
- Bunchman T. E., Brookshire C. A. Cyclosporine-induced synthesis of endothelin by cultured human endothelial cells. J Clin Invest. 1991 Jul;88(1):310–314. doi: 10.1172/JCI115293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damian R. T. Immunity in schistosomiasis: a holistic view. Contemp Top Immunobiol. 1984;12:359–420. doi: 10.1007/978-1-4684-4571-8_10. [DOI] [PubMed] [Google Scholar]
- Emeson E. E., Robertson A. L., Jr T lymphocytes in aortic and coronary intimas. Their potential role in atherogenesis. Am J Pathol. 1988 Feb;130(2):369–376. [PMC free article] [PubMed] [Google Scholar]
- Evans G. O. Hypomagnesaemia, hypoalbuminaemia and plasma lipid changes in rats following the oral administration of ciclosporin. Comp Biochem Physiol C. 1988;89(2):375–376. doi: 10.1016/0742-8413(88)90240-x. [DOI] [PubMed] [Google Scholar]
- Ferns G. A., Reidy M. A., Ross R. Balloon catheter de-endothelialization of the nude rat carotid. Response to injury in the absence of functional T lymphocytes. Am J Pathol. 1991 Apr;138(4):1045–1057. [PMC free article] [PubMed] [Google Scholar]
- Ferns G., Reidy M., Ross R. Vascular effects of cyclosporine A in vivo and in vitro. Am J Pathol. 1990 Aug;137(2):403–413. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A. Lymphokine gene expression in vivo is inhibited by cyclosporin A. J Exp Med. 1990 Feb 1;171(2):533–544. doi: 10.1084/jem.171.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen H. J., Bendtsen T. F., Korbo L., Marcussen N., Møller A., Nielsen K., Nyengaard J. R., Pakkenberg B., Sørensen F. B., Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988 May;96(5):379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Holm S., Fotev Z., Hedrich H. J., Fingerle J. T lymphocytes inhibit the vascular response to injury. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10530–10534. doi: 10.1073/pnas.88.23.10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989 Jul;135(1):169–175. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Seifert P. S., Olsson G., Bondjers G. Immunohistochemical detection of macrophages and T lymphocytes in atherosclerotic lesions of cholesterol-fed rabbits. Arterioscler Thromb. 1991 May-Jun;11(3):745–750. doi: 10.1161/01.atv.11.3.745. [DOI] [PubMed] [Google Scholar]
- Ishida O., Ochi M., Miyamoto Y., Kuta Y., Akiyama M. Suppression by cyclosporine of cellular and humoral reactivity after peripheral nerve allografts in mice. Transplantation. 1989 Nov;48(5):824–829. doi: 10.1097/00007890-198911000-00020. [DOI] [PubMed] [Google Scholar]
- Jevnikar A. M., Petric R., Holub B. J., Philbrick D. J., Clark W. F. Effect of cyclosporine on plasma lipids and modification with dietary fish oil. Transplantation. 1988 Nov;46(5):722–725. doi: 10.1097/00007890-198811000-00018. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Hansson G. K. Cyclosporin A inhibits smooth muscle proliferation in the vascular response to injury. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2303–2306. doi: 10.1073/pnas.85.7.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Kahan B. D. Cyclosporine. N Engl J Med. 1989 Dec 21;321(25):1725–1738. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- Kaibara N., Hotokebuchi T., Takagishi K., Katsuki I. Paradoxical effects of cyclosporin A on collagen arthritis in rats. J Exp Med. 1983 Dec 1;158(6):2007–2015. doi: 10.1084/jem.158.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-Virella M. F., Stone P., Ellis S., Colwell J. A. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977 May;23(5):882–884. [PubMed] [Google Scholar]
- Lustig S., Stern N., Golub M. S., Eggena P., Barrett J., Lee D. B. Experimental cyclosporin hypertension: characterization of the rat model. Transplant Proc. 1989 Feb;21(1 Pt 1):950–951. [PubMed] [Google Scholar]
- Metzger J. M., Peterson L. B. Cyclosporin A enhances the pulmonary granuloma response induced by Schistosoma mansoni eggs. Immunopharmacology. 1988 Mar-Apr;15(2):103–115. doi: 10.1016/0162-3109(88)90057-4. [DOI] [PubMed] [Google Scholar]
- Munro J. M., van der Walt J. D., Munro C. S., Chalmers J. A., Cox E. L. An immunohistochemical analysis of human aortic fatty streaks. Hum Pathol. 1987 Apr;18(4):375–380. doi: 10.1016/s0046-8177(87)80168-5. [DOI] [PubMed] [Google Scholar]
- Nguyen D. T., Eskandai M. K., DeForge L. E., Raiford C. L., Strieter R. M., Kunkel S. L., Remick D. G. Cyclosporin a modulation of tumor necrosis factor gene expression and effects in vitro and in vivo. J Immunol. 1990 May 15;144(10):3822–3828. [PubMed] [Google Scholar]
- Norin A. J., Emeson E. E. Effects of restoring lethally irradiated mice with anti-Thy 1.2-treated bone marrow: graft-vs-host, host-vs-graft, and mitogen reactivity. J Immunol. 1978 Mar;120(3):754–758. [PubMed] [Google Scholar]
- Paigen B., Morrow A., Holmes P. A., Mitchell D., Williams R. A. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987 Dec;68(3):231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- Reisman L., Cooper D., Lieberman K. V., Martinelli G. P. Cyclosporine suppresses IL-1 production by isolated human monocytes and by the human histiocytoma cell line, THP-1. Transplant Proc. 1990 Aug;22(4):1744–1746. [PubMed] [Google Scholar]
- Rotolo F. S., Branum G. D., Bowers B. A., Meyers W. C. Effect of cyclosporine on bile secretion in rats. Am J Surg. 1986 Jan;151(1):35–40. doi: 10.1016/0002-9610(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Sakaguchi N. Organ-specific autoimmune disease induced in mice by elimination of T cell subsets. V. Neonatal administration of cyclosporin A causes autoimmune disease. J Immunol. 1989 Jan 15;142(2):471–480. [PubMed] [Google Scholar]
- Schwinzer R., Hedrich H. J., Wonigeit K. T cell differentiation in athymic nude rats (rnu/rnu): demonstration of a distorted T cell subset structure by flow cytometry analysis. Eur J Immunol. 1989 Oct;19(10):1841–1847. doi: 10.1002/eji.1830191013. [DOI] [PubMed] [Google Scholar]
- Schwinzer R., Hedrich H. J., Wonigeit K. The alloreactive potential of T-like cells from athymic nude rats (rnu/rnu). Transplant Proc. 1987 Feb;19(1 Pt 1):285–287. [PubMed] [Google Scholar]
- Stamler J. S., Vaughan D. E., Rudd M. A., Mudge G. H., Kirshenbaum J., Young P., Alexander R. W., Loscalzo J. Frequency of hypercholesterolemia after cardiac transplantation. Am J Cardiol. 1988 Dec 1;62(17):1268–1272. doi: 10.1016/0002-9149(88)90272-x. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Hansson G. K. Cyclosporine A inhibits induction of DNA synthesis by PDGF and other peptide mitogens in cultured rat aortic smooth muscle cells and dermal fibroblasts. Growth Factors. 1991;4(3):209–219. doi: 10.3109/08977199109104817. [DOI] [PubMed] [Google Scholar]
- Wick G., Müller P. U., Schwarz S. Effect of cyclosporin A on spontaneous autoimmune thyroiditis of Obese strain (OS) chickens. Eur J Immunol. 1982 Oct;12(10):877–881. doi: 10.1002/eji.1830121014. [DOI] [PubMed] [Google Scholar]
- Xu Q. B., Oberhuber G., Gruschwitz M., Wick G. Immunology of atherosclerosis: cellular composition and major histocompatibility complex class II antigen expression in aortic intima, fatty streaks, and atherosclerotic plaques in young and aged human specimens. Clin Immunol Immunopathol. 1990 Sep;56(3):344–359. doi: 10.1016/0090-1229(90)90155-j. [DOI] [PubMed] [Google Scholar]
- Zoja C., Furci L., Ghilardi F., Zilio P., Benigni A., Remuzzi G. Cyclosporin-induced endothelial cell injury. Lab Invest. 1986 Oct;55(4):455–462. [PubMed] [Google Scholar]
- de Groen P. C. Cyclosporine, low-density lipoprotein, and cholesterol. Mayo Clin Proc. 1988 Oct;63(10):1012–1021. doi: 10.1016/s0025-6196(12)64916-7. [DOI] [PubMed] [Google Scholar]
- van der Wal A. C., Das P. K., Bentz van de Berg D., van der Loos C. M., Becker A. E. Atherosclerotic lesions in humans. In situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest. 1989 Aug;61(2):166–170. [PubMed] [Google Scholar]