Abstract
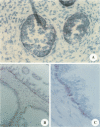
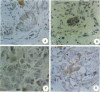
The oncoprotein encoded by bc1-2 is unique because of its intracellular location (a mitochondrial membrane protein) and apparent mode of action (suppression of apoptosis). To date, this oncogene has been associated only with the development of certain forms of human B-cell lymphoma. In this report, we describe our experience with a monoclonal antibody made against a synthetic peptide for bc1-2 that can recognize the bc1-2 protein and identify cells in human prostate glands expressing this proto-oncogene with in situ immunohistochemical procedures. These procedures were utilized to survey a series of 62 human tissues to evaluate whether bc1-2 might have a role in the developing prostate gland or in prostate oncogenesis. While all primordial epithelial cells in a fetal prostate gland immunostain for bc1-2, normal and hypertrophic prostate glands of the adult show bc1-2 expression restricted to the basal cells. All epithelial cells in areas of prostatic intraepithelial neoplasia were stained by this antibody, as were most (62%) localized invasive prostatic carcinomas. In contrast, all primary prostatic carcinomas and metastases obtained from metastatic prostate cancer patients after hormone treatment (hormone-refractory tumors) stained positive for bc1-2. This study demonstrates that the oncoprotein encoded by bc1-2 can be detected at sequential stages in the natural history of human prostate cancer. Since the bc1-2 oncoprotein is known to suppress the cellular response to apoptotic stimuli, it will be important to determine whether bc1-2 expression is a factor in the development of prostate cancers and in the survival of hormone-refractory prostate cancer cells.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhshi A., Jensen J. P., Goldman P., Wright J. J., McBride O. W., Epstein A. L., Korsmeyer S. J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985 Jul;41(3):899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Bookstein R., Rio P., Madreperla S. A., Hong F., Allred C., Grizzle W. E., Lee W. H. Promoter deletion and loss of retinoblastoma gene expression in human prostate carcinoma. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7762–7766. doi: 10.1073/pnas.87.19.7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring C. C., Squires T. S., Tong T. Cancer statistics, 1992. CA Cancer J Clin. 1992 Jan-Feb;42(1):19–38. doi: 10.3322/canjclin.42.1.19. [DOI] [PubMed] [Google Scholar]
- Brawer M. K., Bigler S. A., Sohlberg O. E., Nagle R. B., Lange P. H. Significance of prostatic intraepithelial neoplasia on prostate needle biopsy. Urology. 1991 Aug;38(2):103–107. doi: 10.1016/s0090-4295(05)80067-6. [DOI] [PubMed] [Google Scholar]
- Brawer M. K. Prostatic intraepithelial neoplasia: a premalignant lesion. Hum Pathol. 1992 Mar;23(3):242–248. doi: 10.1016/0046-8177(92)90104-b. [DOI] [PubMed] [Google Scholar]
- Brothman A. R., Peehl D. M., Patel A. M., McNeal J. E. Frequency and pattern of karyotypic abnormalities in human prostate cancer. Cancer Res. 1990 Jun 15;50(12):3795–3803. [PubMed] [Google Scholar]
- Buttyan R., Sawczuk I. S., Benson M. C., Siegal J. D., Olsson C. A. Enhanced expression of the c-myc protooncogene in high-grade human prostate cancers. Prostate. 1987;11(4):327–337. doi: 10.1002/pros.2990110405. [DOI] [PubMed] [Google Scholar]
- Carter B. S., Ewing C. M., Ward W. S., Treiger B. F., Aalders T. W., Schalken J. A., Epstein J. I., Isaacs W. B. Allelic loss of chromosomes 16q and 10q in human prostate cancer. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8751–8755. doi: 10.1073/pnas.87.22.8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Levy Z., Nourse J., Cleary M. L. The bcl-2 candidate proto-oncogene product is a 24-kilodalton integral-membrane protein highly expressed in lymphoid cell lines and lymphomas carrying the t(14;18) translocation. Mol Cell Biol. 1989 Feb;9(2):701–710. doi: 10.1128/mcb.9.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Kramer S. A., Spahr J., Brendler C. B., Glenn J. F., Paulson D. F. Experience with Gleason's histopathologic grading in prostatic cancer. J Urol. 1980 Aug;124(2):223–225. doi: 10.1016/s0022-5347(17)55381-1. [DOI] [PubMed] [Google Scholar]
- Kyprianou N., English H. F., Isaacs J. T. Programmed cell death during regression of PC-82 human prostate cancer following androgen ablation. Cancer Res. 1990 Jun 15;50(12):3748–3753. [PubMed] [Google Scholar]
- Lundgren R., Mandahl N., Heim S., Limon J., Henrikson H., Mitelman F. Cytogenetic analysis of 57 primary prostatic adenocarcinomas. Genes Chromosomes Cancer. 1992 Jan;4(1):16–24. doi: 10.1002/gcc.2870040103. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Nunez G., Platt F. M., Hockenberry D., London L., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2-immunoglobulin transgene expands a resting but responsive immunoglobulin M and D-expressing B-cell population. Mol Cell Biol. 1990 May;10(5):1901–1907. doi: 10.1128/mcb.10.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton R. A., Steiner M. S., Walsh P. C. Cancer control following anatomical radical prostatectomy: an interim report. J Urol. 1991 Jun;145(6):1197–1200. doi: 10.1016/s0022-5347(17)38574-9. [DOI] [PubMed] [Google Scholar]
- Nagle R. B., Brawer M. K., Kittelson J., Clark V. Phenotypic relationships of prostatic intraepithelial neoplasia to invasive prostatic carcinoma. Am J Pathol. 1991 Jan;138(1):119–128. [PMC free article] [PubMed] [Google Scholar]
- Parmiter A. H., Balaban G., Herlyn M., Clark W. H., Jr, Nowell P. C. A t(1;19) chromosome translocation in three cases of human malignant melanoma. Cancer Res. 1986 Mar;46(3):1526–1529. [PubMed] [Google Scholar]
- Pezzella F., Tse A. G., Cordell J. L., Pulford K. A., Gatter K. C., Mason D. Y. Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990 Aug;137(2):225–232. [PMC free article] [PubMed] [Google Scholar]
- Rouleau M., Léger J., Tenniswood M. Ductal heterogeneity of cytokeratins, gene expression, and cell death in the rat ventral prostate. Mol Endocrinol. 1990 Dec;4(12):2003–2013. doi: 10.1210/mend-4-12-2003. [DOI] [PubMed] [Google Scholar]
- Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Seto M., Jaeger U., Hockett R. D., Graninger W., Bennett S., Goldman P., Korsmeyer S. J. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988 Jan;7(1):123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman G. A., Green E. D., Young R. L., Jockel J. I., Domer P. H., Korsmeyer S. J. Meiotic recombination between yeast artificial chromosomes yields a single clone containing the entire BCL2 protooncogene. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9913–9917. doi: 10.1073/pnas.87.24.9913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogani P. C., Fair W. R. Treatment of advanced prostatic cancer. Urol Clin North Am. 1987 May;14(2):353–371. [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991 Nov 29;67(5):889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Gorham J., Cossman J., Jaffe E., Croce C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985 Sep 27;229(4720):1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Viola M. V., Fromowitz F., Oravez S., Deb S., Finkel G., Lundy J., Hand P., Thor A., Schlom J. Expression of ras oncogene p21 in prostate cancer. N Engl J Med. 1986 Jan 16;314(3):133–137. doi: 10.1056/NEJM198601163140301. [DOI] [PubMed] [Google Scholar]
- Ware J. L., Maygarden S. J., Koontz W. W., Jr, Strom S. C. Immunohistochemical detection of c-erbB-2 protein in human benign and neoplastic prostate. Hum Pathol. 1991 Mar;22(3):254–258. doi: 10.1016/0046-8177(91)90159-m. [DOI] [PubMed] [Google Scholar]