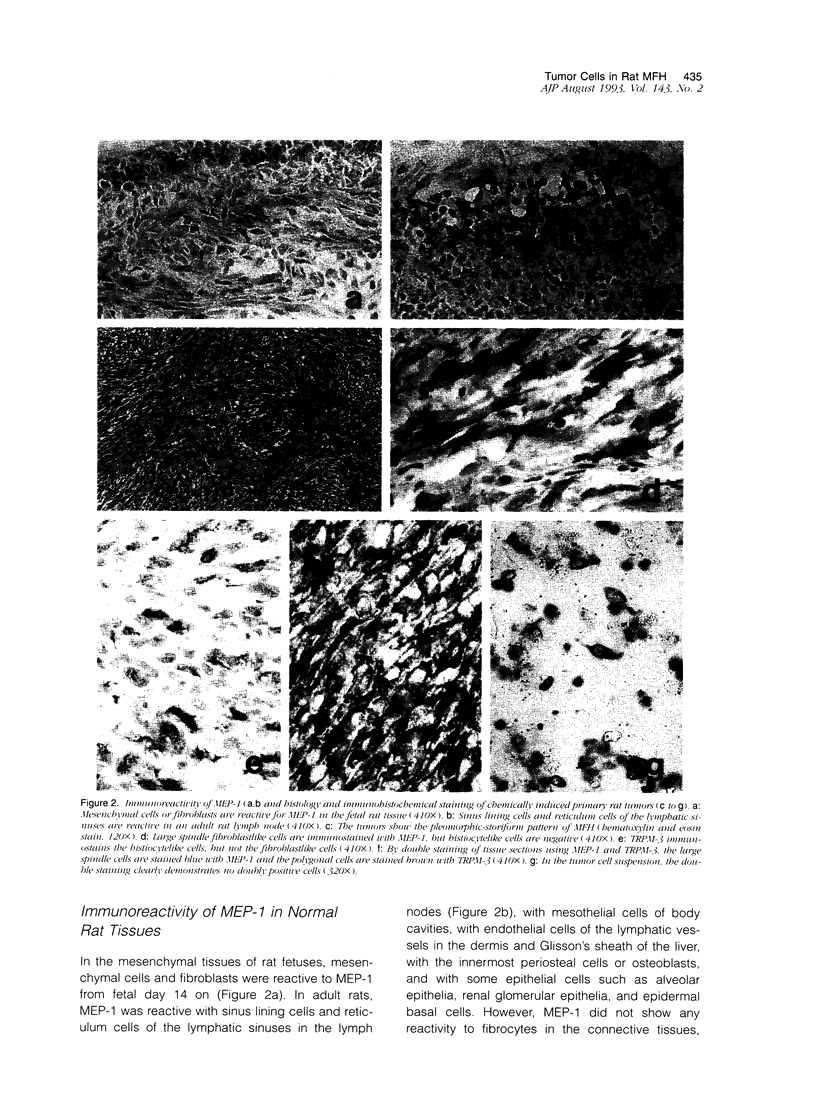
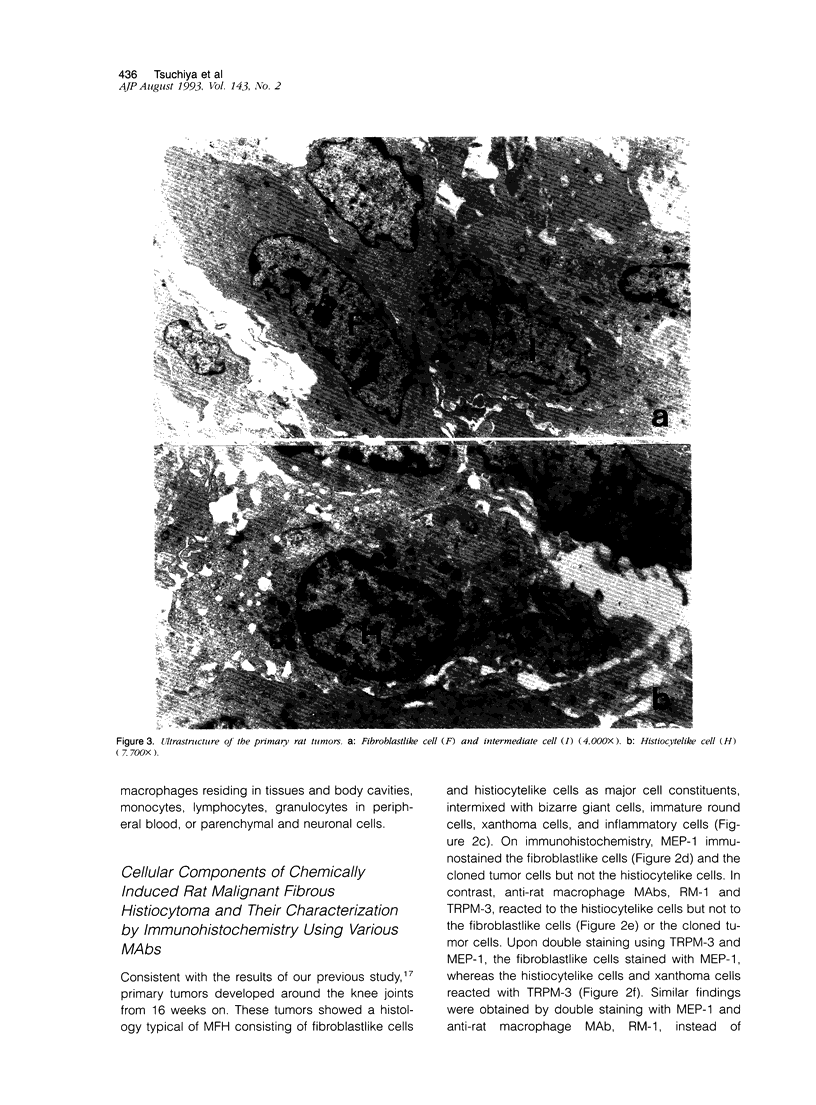
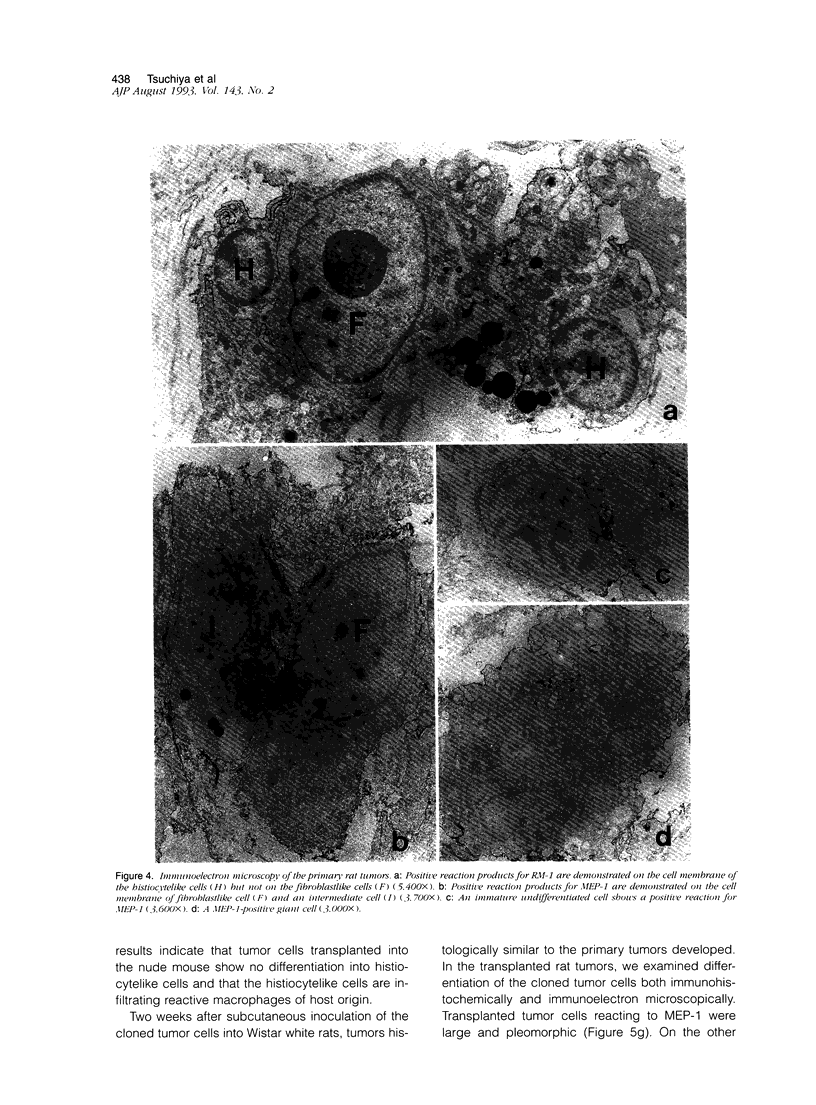
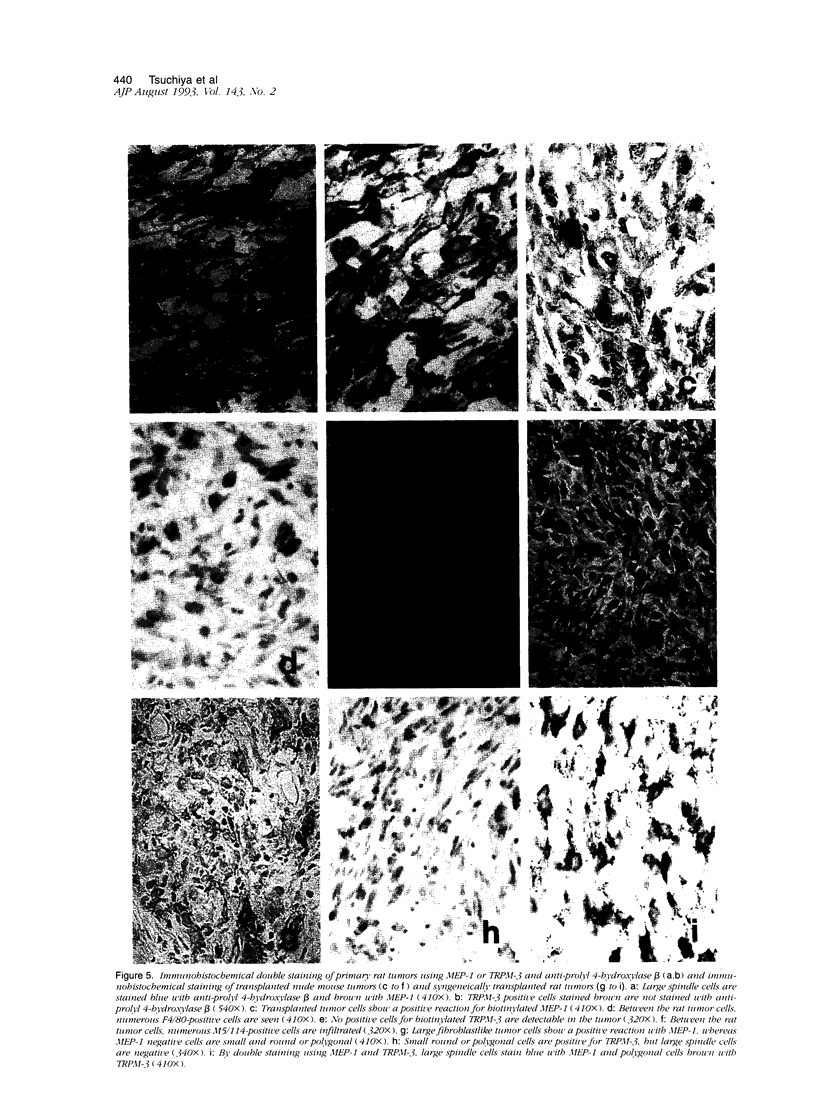
Abstract
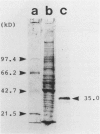
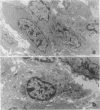
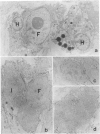
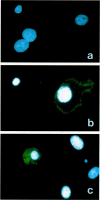
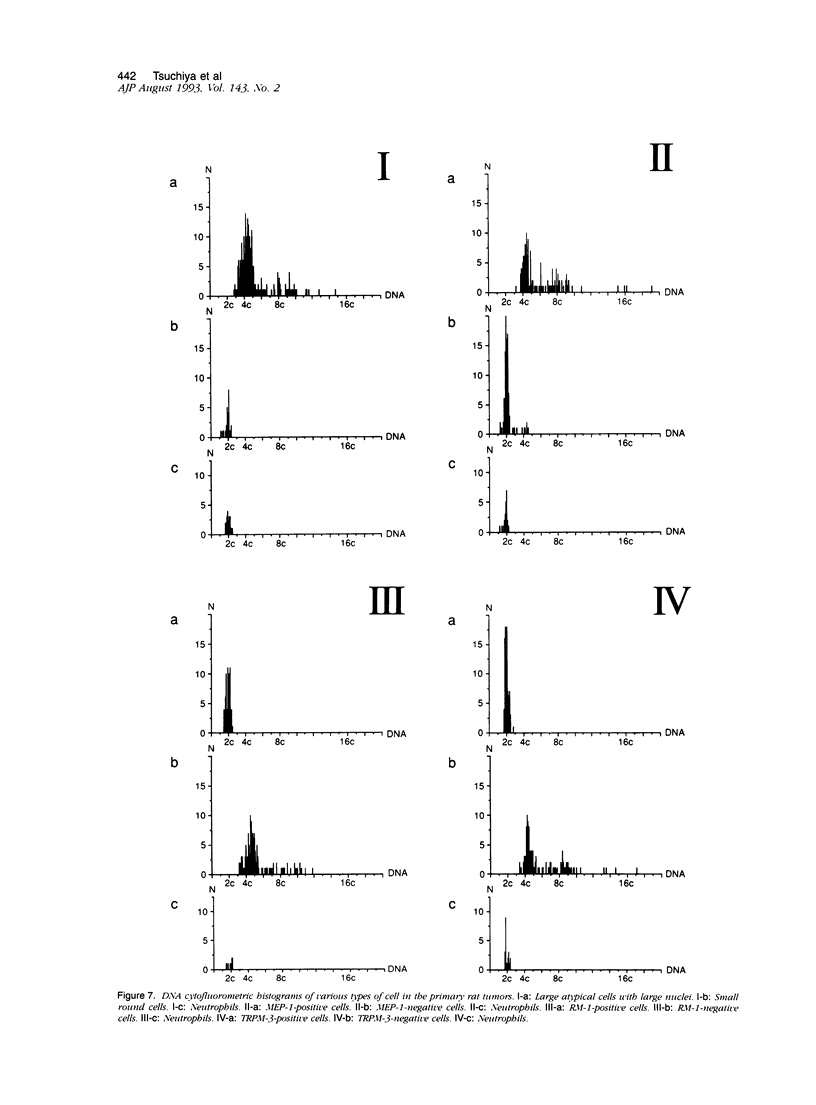
Malignant fibrous histiocytoma (MFH) was induced in rats by injection of 9,10-dimethyl-1,2-benzanthracene. Using cell suspensions prepared from the heterotransplanted nude mouse tumor as immunogen, a monoclonal antibody, (MAb), MEP-1, against fibroblastlike MFH tumor cells was generated. In the primary rat tumors and transplanted rat or nude mouse tumors, MEP-1 reacted specifically with the fibroblastlike cells but not with the histiocytelike cells or xanthoma cells. Anti-rat macrophage MAbs RM-1 and TRPM-3 did not stain the fibroblastlike cells, but both were reactive with the histiocytelike cells. Double stainings with both MEP-1 and RM-1 or TRPM-3 did not detect any double positive cells. Immunoelectron microscopy using these MAbs showed that the fibroblastlike cells were the major cell component of the primary and transplanted rat tumors and that their cell membrane was stained positively with MEP-1, but not for RM-1 or TRPM-3. By the double staining method using a MAb against prolyl 4-hydroxylase beta and MEP-1 or TRPM-3, this enzyme was demonstrated in MEP-1-positive cells but not in TRPM-3-positive cells. Results obtained by DNA cytofluorometry with 4,6-diamidino-2-phenylindole dihydrochloride staining or by the combined method of DNA cytofluorometry and indirect immunofluorescence, using MEP-1, RM-1, and TRPM-3, indicate that MEP-1-positive cells are neoplastic cells of rat MFH having proliferation activity. In the transplanted nude mouse tumors, no differentiation of MEP-1-positive rat tumor cells into histiocytelike cells was detected, and all histiocytelike cells were immunostained by F4/80 and most of them were positive for M5/114. These results suggest that fibroblastlike cells and intermediate cells are tumor cells of 9,10-dimethyl-1,2-benzanthracene-induced rat MFH showing differentiation toward fibroblasts and that histiocytelike cells are infiltrated macrophages.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alguacil-Garcia A., Unni K. K., Goellner J. R. Malignant fibrous histiocytoma: an ultrastructural study of six cases. Am J Clin Pathol. 1978 Feb;69(2):121–129. doi: 10.1093/ajcp/69.2.121. [DOI] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Bai Y., Muragaki Y., Obata K., Iwata K., Ooshima A. Immunological properties of monoclonal antibodies to human and rat prolyl 4-hydroxylase. J Biochem. 1986 Jun;99(6):1563–1570. doi: 10.1093/oxfordjournals.jbchem.a135629. [DOI] [PubMed] [Google Scholar]
- Bartal A. H., Lichtig C., Cardo C. C., Feit C., Robinson E., Hirshaut Y. Monoclonal antibody defining fibroblasts appearing in fetal and neoplastic tissues. J Natl Cancer Inst. 1986 Mar;76(3):415–421. [PubMed] [Google Scholar]
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Brecher M. E., Franklin W. A. Absence of mononuclear phagocyte antigens in malignant fibrous histiocytoma. Am J Clin Pathol. 1986 Sep;86(3):344–348. doi: 10.1093/ajcp/86.3.344. [DOI] [PubMed] [Google Scholar]
- Brown J. M. Detection of a human sarcoma-associated antigen with monoclonal antibodies. Cancer Res. 1983 May;43(5):2113–2120. [PubMed] [Google Scholar]
- Bruland O., Fodstad O., Funderud S., Pihl A. New monoclonal antibodies specific for human sarcomas. Int J Cancer. 1986 Jul 15;38(1):27–31. doi: 10.1002/ijc.2910380106. [DOI] [PubMed] [Google Scholar]
- Fu Y. S., Gabbiani G., Kaye G. I., Lattes R. Malignant soft tissue tumors of probable histiocytic origin (malignant fibrous histiocytomas): general considerations and electron microscopic and tissue culture studies. Cancer. 1975 Jan;35(1):176–198. doi: 10.1002/1097-0142(197501)35:1<176::aid-cncr2820350123>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Fukuda T., Tsuneyoshi M., Enjoji M. Malignant fibrous histiocytoma of soft parts: an ultrastructural quantitative study. Ultrastruct Pathol. 1988 Jan-Feb;12(1):117–129. doi: 10.3109/01913128809048480. [DOI] [PubMed] [Google Scholar]
- Hamada S., Fujita S. DAPI staining improved for quantitative cytofluorometry. Histochemistry. 1983;79(2):219–226. doi: 10.1007/BF00489783. [DOI] [PubMed] [Google Scholar]
- Higashi K., Naito M., Takeya M., Ando M., Araki S., Takahashi K. Ontogenetic development, differentiation, and phenotypic expression of macrophages in fetal rat lungs. J Leukoc Biol. 1992 May;51(5):444–454. doi: 10.1002/jlb.51.5.444. [DOI] [PubMed] [Google Scholar]
- Hoffman M. A., Dickersin G. R. Malignant fibrous histiocytoma: an ultrastructural study of eleven cases. Hum Pathol. 1983 Oct;14(10):913–922. doi: 10.1016/s0046-8177(83)80166-x. [DOI] [PubMed] [Google Scholar]
- Homma W., Wünsch P. H. Experimental-induced sarcomas after intra-articular injection of 9-10-dimethyl-1-2-benzanthracene. Arch Orthop Trauma Surg. 1983;102(2):111–113. doi: 10.1007/BF02498726. [DOI] [PubMed] [Google Scholar]
- Hosokawa Y., Tsuchihashi Y., Okabe H., Toyama M., Namura K., Kuga M., Yonezawa T., Fujita S., Ashihara T. Pleomorphic xanthoastrocytoma. Ultrastructural, immunohistochemical, and DNA cytofluorometric study of a case. Cancer. 1991 Aug 15;68(4):853–859. doi: 10.1002/1097-0142(19910815)68:4<853::aid-cncr2820680430>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Imai Y., Yamakawa M., Sato T., Suda A. Malignant fibrous histiocytoma: similarities to the "fibrohistiocytoid cells" in chronic inflammation. Virchows Arch A Pathol Anat Histopathol. 1989;414(4):285–298. doi: 10.1007/BF00734082. [DOI] [PubMed] [Google Scholar]
- Iwasaki H., Isayama T., Johzaki H., Kikuchi M. Malignant fibrous histiocytoma. Evidence of perivascular mesenchymal cell origin immunocytochemical studies with monoclonal anti-MFH antibodies. Am J Pathol. 1987 Sep;128(3):528–537. [PMC free article] [PubMed] [Google Scholar]
- Katenkamp D., Stiller D. Structural patterns and histological behaviour of experimental sarcomas. I. General considerations, histology and histochemistry. Exp Pathol (Jena) 1975;11(3-4):182–189. doi: 10.1016/s0014-4908(75)80059-7. [DOI] [PubMed] [Google Scholar]
- Kato T., Takeya M., Takagi K., Takahashi K. Chemically induced transplantable malignant fibrous histiocytoma of the rat. Analyses with immunohistochemistry, immunoelectron microscopy and [3H]thymidine autoradiography. Lab Invest. 1990 May;62(5):635–645. [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- O'BRIEN J. E., STOUT A. P. MALIGNANT FIBROUS XANTHOMAS. Cancer. 1964 Nov;17:1445–1455. doi: 10.1002/1097-0142(196411)17:11<1445::aid-cncr2820171112>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- OZZELLO L., STOUT A. P., MURRAY M. R. Cultural characteristics of malignant histiocytomas and fibrous xanthomas. Cancer. 1963 Mar;16:331–344. doi: 10.1002/1097-0142(196303)16:3<331::aid-cncr2820160307>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Radio S. J., Wooldridge T. N., Linder J. Flow cytometric DNA analysis of malignant fibrous histiocytoma and related fibrohistiocytic tumors. Hum Pathol. 1988 Jan;19(1):74–77. doi: 10.1016/s0046-8177(88)80319-8. [DOI] [PubMed] [Google Scholar]
- Roholl P. J., Kleyne J., Van Unnik J. A. Characterization of tumor cells in malignant fibrous histiocytomas and other soft-tissue tumors, in comparison with malignant histiocytes. II. Immunoperoxidase study on cryostat sections. Am J Pathol. 1985 Nov;121(2):269–274. [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K. Malignant fibrous histiocytoma induced by intra-articular injection of 9,10-dimethyl-1,2-benzanthracene in the rat. Pathological and enzyme histochemical studies. Cancer. 1986 Jun 15;57(12):2313–2322. doi: 10.1002/1097-0142(19860615)57:12<2313::aid-cncr2820571213>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Shimokawa Y., Takeya M., Miyauchi Y., Takahashi K. A monoclonal antibody, RbM2, specific for a lysosomal membrane antigen of rabbit monocyte/macrophages. Immunology. 1990 Aug;70(4):513–519. [PMC free article] [PubMed] [Google Scholar]
- Shirasuna K., Sugiyama M., Miyazaki T. Establishment and characterization of neoplastic cells from a malignant fibrous histiocytoma. A possible stem cell line. Cancer. 1985 Jun 1;55(11):2521–2532. doi: 10.1002/1097-0142(19850601)55:11<2521::aid-cncr2820551102>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Soini Y., Autio-Harmainen H. Tumor cells of malignant fibrous histiocytomas express mRNA for laminin. Am J Pathol. 1991 Nov;139(5):1061–1068. [PMC free article] [PubMed] [Google Scholar]
- Stastny J. J., Brown J. M., Beattie C. W., Das Gupta T. K. Monoclonal antibody identification and characterization of two human sarcoma-associated antigens. Cancer Res. 1991 Jul 15;51(14):3768–3773. [PubMed] [Google Scholar]
- Strauchen J. A., Dimitriu-Bona A. Malignant fibrous histiocytoma. Expression of monocyte/macrophage differentiation antigens detected with monoclonal antibodies. Am J Pathol. 1986 Aug;124(2):303–309. [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Takahashi H., Naito M., Sato T., Kojima M. Ultrastructural and functional development of macrophages in the dermal tissue of rat fetuses. Cell Tissue Res. 1983;232(3):539–552. doi: 10.1007/BF00216427. [DOI] [PubMed] [Google Scholar]
- Takeya M., Hsiao L., Shimokawa Y., Takahashi K. Heterogeneity of rat macrophages recognized by monoclonal antibodies: an immunohistochemical and immunoelectron microscopic study. J Histochem Cytochem. 1989 May;37(5):635–641. doi: 10.1177/37.5.2649558. [DOI] [PubMed] [Google Scholar]
- Takeya M., Hsiao L., Takahashi K. A new monoclonal antibody, TRPM-3, binds specifically to certain rat macrophage populations: immunohistochemical and immunoelectron microscopic analysis. J Leukoc Biol. 1987 Mar;41(3):187–195. doi: 10.1002/jlb.41.3.187. [DOI] [PubMed] [Google Scholar]
- Takeya M., Yoshimura T., Leonard E. J., Kato T., Okabe H., Takahashi K. Production of monocyte chemoattractant protein-1 by malignant fibrous histiocytoma: relation to the origin of histiocyte-like cells. Exp Mol Pathol. 1991 Feb;54(1):61–71. doi: 10.1016/0014-4800(91)90044-x. [DOI] [PubMed] [Google Scholar]
- Ukai K., Terashima K., Imai Y., Shinzawa H., Okuyama Y., Takahashi T., Ishikawa M. Proliferation kinetics of rat Kupffer cells after partial hepatectomy. Immunohistochemical and ultrastructural analysis. Acta Pathol Jpn. 1990 Sep;40(9):623–634. doi: 10.1111/j.1440-1827.1990.tb01610.x. [DOI] [PubMed] [Google Scholar]
- Weiss S. W., Enzinger F. M. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer. 1978 Jun;41(6):2250–2266. doi: 10.1002/1097-0142(197806)41:6<2250::aid-cncr2820410626>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Takeya M., Takahashi K. Molecular cloning of rat monocyte chemoattractant protein-1 (MCP-1) and its expression in rat spleen cells and tumor cell lines. Biochem Biophys Res Commun. 1991 Jan 31;174(2):504–509. doi: 10.1016/0006-291x(91)91445-i. [DOI] [PubMed] [Google Scholar]
- Yumoto T., Morimoto K. Experimental approach to fibrous histiocytoma. Acta Pathol Jpn. 1980 Sep;30(5):767–778. doi: 10.1111/j.1440-1827.1980.tb00972.x. [DOI] [PubMed] [Google Scholar]