Abstract
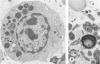
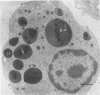
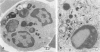
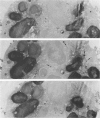
Lysosomal enlargement in Chédiak-Higashi Syndrome (CHS) occurs to varying degrees in different cell types and has provided insight into the pathophysiology of lysosomal granules. This study was undertaken to determine the extent of involvement of eosinophil crystalloid granules (CGs) and smaller non-crystalloid granules (NCGs) in giant granule formation. Eosinophils from two CHS patients were evaluated after glutaraldehyde fixation and staining for morphologic examination, peroxidase, and complex carbohydrate using uranyl acetate-lead citrate, diaminobenzidine-lead citrate, and periodate-thiocarbohydrazide-silver proteinate (PA-TCH-SP) methods, respectively. Although many CGs appeared normal in shape and size, several CGs appeared enlarged and a few measured over 5 microns in diameter, consistent with giant granule formation in CHS. These giant granules either occasionally contained a single large crystalloid or, more frequently, contained numerous normal-size crystalloids. Enlargement of granules was also observed in some precursor CGs of bone marrow early eosinophils, indicating that giant granule formation was initiated during granule genesis. Almost all NCGs in late eosinophils were small granules and stained strongly with PA-TCH-SP in contrast to CGs. Most, but not all small granules were peroxidase-positive in eosinophil precursors, whereas the percentage of peroxidase-negative small granules increased in late eosinophils. This indicated the presence of at least two small granule populations. Morphometric studies indicated CHS selectively involved CGs and demonstrated that neither the average size nor numbers of NCGs were significantly different from normal eosinophils. Thus, these studies indicate that CHS selectively involves CGs, and demonstrate preservation of normal granule size and heterogeneity for NCGs in late eosinophils. These observations suggest that the underlying CHS pathophysiology does not involve all lysosomal subpopulations.
Full text
PDF

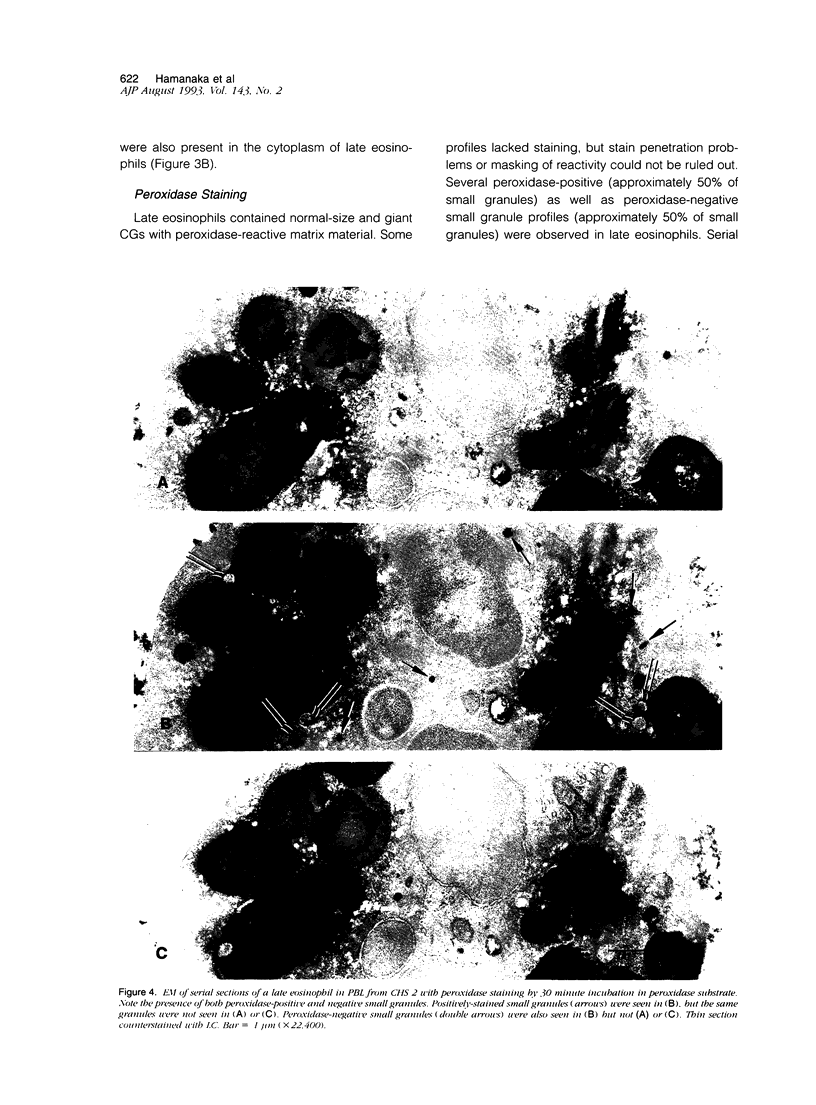

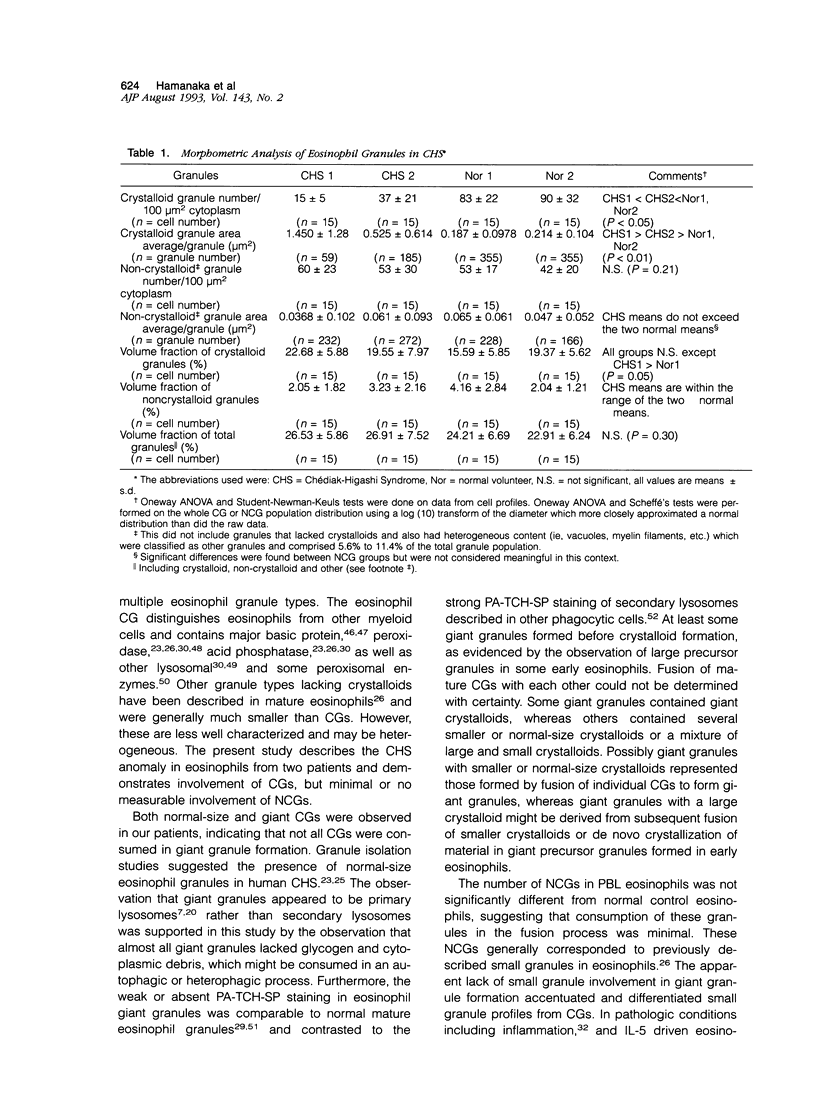
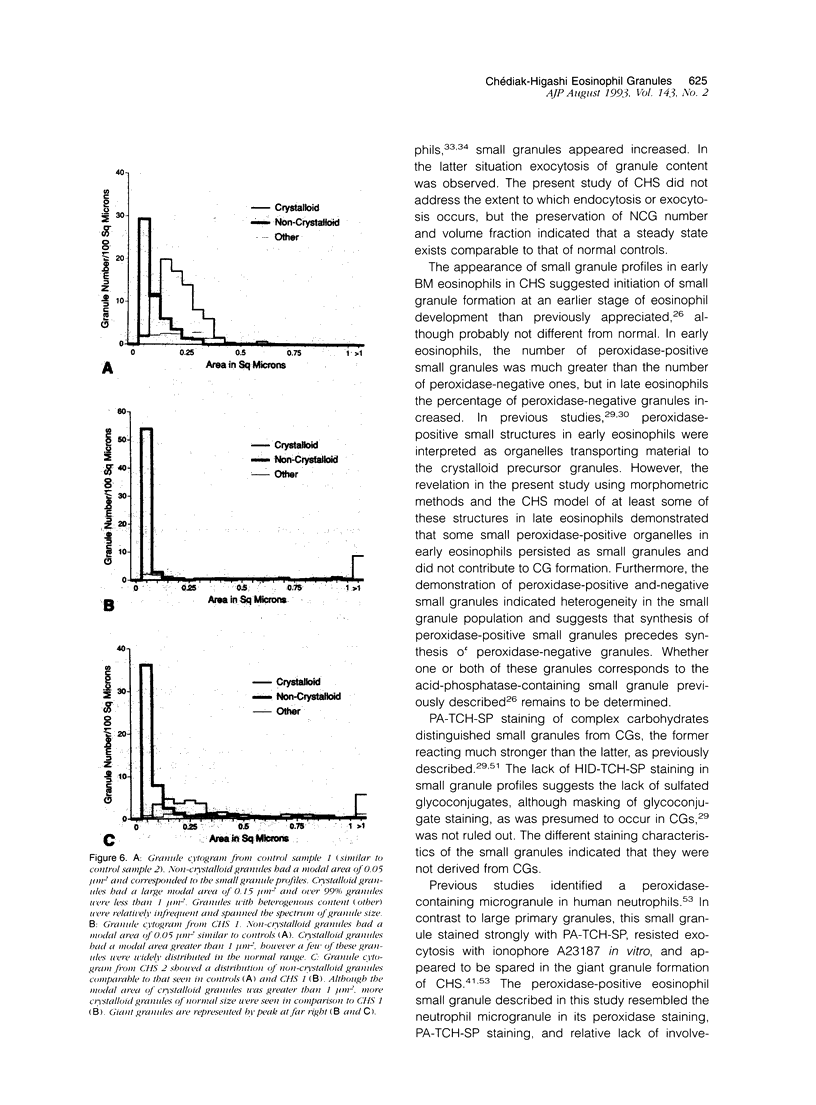


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman G. A., Clark M. A. Ultrastructural localization of peroxidase activity in normal human bone marrow cells. Z Zellforsch Mikrosk Anat. 1971;117(4):463–475. doi: 10.1007/BF00330708. [DOI] [PubMed] [Google Scholar]
- Ayers J. R., Leipold H. W., Padgett G. A. Lesions in Brangus cattle with Chediak-Higashi syndrome. Vet Pathol. 1988 Nov;25(6):432–436. doi: 10.1177/030098588802500605. [DOI] [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Segregation and packaging of granule enzymes in eosinophilic leukocytes. J Cell Biol. 1970 Apr;45(1):54–73. doi: 10.1083/jcb.45.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Y., Nir E. Chediak-Higashi syndrome. Am J Pediatr Hematol Oncol. 1987 Spring;9(1):42–55. doi: 10.1097/00043426-198721000-00008. [DOI] [PubMed] [Google Scholar]
- Blume R. S., Wolff S. M. The Chediak-Higashi syndrome: studies in four patients and a review of the literature. Medicine (Baltimore) 1972 Jul;51(4):247–280. [PubMed] [Google Scholar]
- CHEDIAK M. M. Nouvelle anomalie leucocytaire de caractère constitutionnel et familial. Rev Hematol. 1952;7(3):362–367. [PubMed] [Google Scholar]
- Davis W. C., Douglas S. D. Defective granule formation and function in the Chediak-Higashi syndrome in man and animals. Semin Hematol. 1972 Oct;9(4):431–450. [PubMed] [Google Scholar]
- Davis W. C., Spicer S. S., Greene W. B., Padgett G. A. Ultrastructure of bone marrow granulocytes in normal mink and mink with the homolog of the Chediak-Higashi trait of humans. I. Origin of the abnormal granules present in the neutrophils of mink with the C-HS trait. Lab Invest. 1971 Apr;24(4):303–317. [PubMed] [Google Scholar]
- Davis W. C., Spicer S. S., Greene W. B., Padgett G. A. Ultrastructure of cells in bone marrow and peripheral blood of normal mink and mink with the homologue of the Chediak-Higashi trait of humans. II. Cytoplasmic granules in eosinophils, basophils, mononuclear cells and platelets. Am J Pathol. 1971 Jun;63(3):411–432. [PMC free article] [PubMed] [Google Scholar]
- Dvorak A. M., Ackerman S. J., Furitsu T., Estrella P., Letourneau L., Ishizaka T. Mature eosinophils stimulated to develop in human-cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. II. Vesicular transport of specific granule matrix peroxidase, a mechanism for effecting piecemeal degranulation. Am J Pathol. 1992 Apr;140(4):795–807. [PMC free article] [PubMed] [Google Scholar]
- Dvorak A. M., Furitsu T., Letourneau L., Ishizaka T., Ackerman S. J. Mature eosinophils stimulated to develop in human cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. Part I. Piecemeal degranulation of specific granules and distribution of Charcot-Leyden crystal protein. Am J Pathol. 1991 Jan;138(1):69–82. [PMC free article] [PubMed] [Google Scholar]
- Efrati P., Danon D. Electron-microscopical study of bone marrow cells in a case of Chediak-Higashi-Steinbrinck syndrome. Br J Haematol. 1968 Aug;15(2):173–176. doi: 10.1111/j.1365-2141.1968.tb01526.x. [DOI] [PubMed] [Google Scholar]
- Eguchi M., Komiyama A., Hanamura K., Tsukada M., Akabane T. Electron microscopic observations of leukocytes from an infant with Chediak-Higashi syndrome-cytochemistry and phagocytic study-. Nihon Ketsueki Gakkai Zasshi. 1975 Feb;38(1):27–40. [PubMed] [Google Scholar]
- Fagerland J. A., Hagemoser W. A., Ireland W. P. Ultrastructure and stereology of leukocytes and platelets of normal foxes and a fox with a Chediak-Higashi-like syndrome. Vet Pathol. 1987 Mar;24(2):164–169. doi: 10.1177/030098588702400210. [DOI] [PubMed] [Google Scholar]
- Fittschen C., Parmley R. T., Austin R. L., Crist W. M. Vicinal glycol-staining identifies secondary granules in human normal and Chédiak-Higashi neutrophils. Anat Rec. 1983 Mar;205(3):301–311. doi: 10.1002/ar.1092050307. [DOI] [PubMed] [Google Scholar]
- Gale P. F., Parkin J. L., Quie P. G., Pettit R. E., Nelson R. P., Brunning R. D. Leukocyte granulation abnormality associated with normal neutrophil function and neurologic impairment. Am J Clin Pathol. 1986 Jul;86(1):33–49. doi: 10.1093/ajcp/86.1.33. [DOI] [PubMed] [Google Scholar]
- Gilbert C. S., Parmley R. T., Rice W. G., Kinkade J. M., Jr Heterogeneity of peroxidase-positive granules in normal human an Chédiak-Higashi neutrophils. J Histochem Cytochem. 1993 Jun;41(6):837–849. doi: 10.1177/41.6.8315276. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Adolphson C. R. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- HIGASHI O. Congenital gigantism of peroxidase granules; the first case ever reported of qualitative abnormity of peroxidase. Tohoku J Exp Med. 1954 Feb 25;59(3):315–332. doi: 10.1620/tjem.59.315. [DOI] [PubMed] [Google Scholar]
- Hardin J. H., Spicer S. S. An ultrastructural study of human eosinophil granules: maturational stages and pyroantimonate reactive cation. Am J Anat. 1970 Jul;128(3):283–310. doi: 10.1002/aja.1001280303. [DOI] [PubMed] [Google Scholar]
- Henderson W. R., Chi E. Y. Ultrastructural characterization and morphometric analysis of human eosinophil degranulation. J Cell Sci. 1985 Feb;73:33–48. doi: 10.1242/jcs.73.1.33. [DOI] [PubMed] [Google Scholar]
- Komiyama A., Spicer S. S. Microendocytosis in eosinophilic leukocytes. J Cell Biol. 1975 Mar;64(3):622–635. doi: 10.1083/jcb.64.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. W., Davis W. C., Prieur D. J. The Chediak-Higashi syndrome of cats. Lab Invest. 1977 May;36(5):554–562. [PubMed] [Google Scholar]
- LEADER R. W., PADGETT G. A., GORHAM J. R. STUDIES OF ABNORMAL LEUKOCYTE BODIES IN THE MINK. Blood. 1963 Oct;22:477–484. [PubMed] [Google Scholar]
- Lutzner M. A., Lowrie C. T., Jordan H. W. Giant granules in leukocytes of the beige mouse. J Hered. 1967 Nov-Dec;58(6):299–300. doi: 10.1093/oxfordjournals.jhered.a107620. [DOI] [PubMed] [Google Scholar]
- Marshall T., Shult P., Busse W. W. Release of lysosomal enzyme beta-glucuronidase from isolated human eosinophils. J Allergy Clin Immunol. 1988 Oct;82(4):550–555. doi: 10.1016/0091-6749(88)90964-5. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Inoue M., Nakano T., Nishikawa T., Miyamoto M., Kobayashi T., Kitamura Y. Beige rat: a new animal model of Chediak-Higashi syndrome. Blood. 1989 Jul;74(1):270–273. [PubMed] [Google Scholar]
- Oliver C., Essner E. Formation of anomalous lysosomes in monocytes, neutrophils, and eosinophils from bone marrow of mice with Chédiak-Higashi syndrome. Lab Invest. 1975 Jan;32(1):17–27. [PubMed] [Google Scholar]
- PADGETT G. A., LEADER R. W., GORHAM J. R., O'MARY C. C. THE FAMILIAL OCCURRENCE OF THE CHEDIAK-HIGASHI SYNDROME IN MINK AND CATTLE. Genetics. 1964 Mar;49:505–512. doi: 10.1093/genetics/49.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmley R. T., Eguchi M., Spicer S. S. Ultrastructural cytochemistry of complex carbohydrates in leukocyte granules. J Histochem Cytochem. 1979 Aug;27(8):1167–1170. doi: 10.1177/27.8.90076. [DOI] [PubMed] [Google Scholar]
- Parmley R. T., Rice W. G., Kinkade J. M., Jr, Gilbert C., Barton J. C. Peroxidase-containing microgranules in human neutrophils: physical, morphological, cytochemical, and secretory properties. Blood. 1987 Nov;70(5):1630–1638. [PubMed] [Google Scholar]
- Parmley R. T., Spicer S. S. Altered tissue eosinophils in Hodgkin's Disease. Exp Mol Pathol. 1975 Aug;23(1):70–82. doi: 10.1016/0014-4800(75)90007-6. [DOI] [PubMed] [Google Scholar]
- Parmley R. T., Spicer S. S. Cytochemical and ultrastructural identification of a small type granule in human late eosinophils. Lab Invest. 1974 May;30(5):557–567. [PubMed] [Google Scholar]
- Parmley R. T., Takagi M., Spicer S. S., Thrasher A., Denys F. R. Ultrastructural visualization of complex carbohydrates in eosinophilic leukocytes. Am J Anat. 1982 Sep;165(1):53–67. doi: 10.1002/aja.1001650106. [DOI] [PubMed] [Google Scholar]
- Rozenszajn L. A., David E. B., Sela S. B. Large granules and lysosomal fusion in human Chediak-Higashi white blood cells. Acta Haematol. 1977;57(5):279–289. doi: 10.1159/000207892. [DOI] [PubMed] [Google Scholar]
- STOEBER W. STATISTICAL SIZE DISTRIBUTION ANALYSIS. Lab Invest. 1965 Jun;14:892–908. [PubMed] [Google Scholar]
- Sannes P. L., Eguchi M., Spicer S. S. Ultrastructural localization of complex carbohydrates in rat monocytes and peritoneal and alveolar macrophages. Lab Invest. 1979 Aug;41(2):135–143. [PubMed] [Google Scholar]
- Sannes P. L., Spicer S. S., Katsuyama T. Ultrastructural localization of sulfated complex carbohydrates with a modified iron diamine procedure. J Histochem Cytochem. 1979 Jul;27(7):1108–1111. doi: 10.1177/27.7.89157. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Hardin J. H., Setser M. E. Ultrastructural visualization of sulphated complex carbohydrates in blood and epithelial cells with the high iron diamine procedure. Histochem J. 1978 Jul;10(4):435–452. doi: 10.1007/BF01003007. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Sato A., Vincent R., Eguchi M., Poon K. C. Lysosome enlargement in the Chediak-Higashi syndrome. Fed Proc. 1981 Apr;40(5):1451–1455. [PubMed] [Google Scholar]
- Stolz W., Graubner U., Gerstmeier J., Burg G., Belohradsky B. H. Chédiak-Higashi syndrome: approaches in diagnosis and treatment. Curr Probl Dermatol. 1989;18:93–100. doi: 10.1159/000416843. [DOI] [PubMed] [Google Scholar]
- Takagi M., Parmley R. T., Denys F. R. Ultrastructural cytochemistry and radioautography of complex carbohydrates in secretory granules of epiphyseal chondrocytes. Lab Invest. 1981 Feb;44(2):116–126. [PubMed] [Google Scholar]
- West B. C. Chédiak-Higashi syndrome neutrophils are characterized by the absence of both normal azurophilic granules. Am J Pathol. 1986 Jan;122(1):177–189. [PMC free article] [PubMed] [Google Scholar]
- White J. G., Krumwiede M. Normal-sized primary lysosomes are present in Chediak-Higashi syndrome neutrophils. Pediatr Res. 1987 Aug;22(2):208–215. doi: 10.1203/00006450-198708000-00022. [DOI] [PubMed] [Google Scholar]
- White J. G. The Chediak-Higashi syndrome. Fine structure of giant inclusions in freeze-fractured neutrophils. Am J Pathol. 1973 Sep;72(3):503–520. [PMC free article] [PubMed] [Google Scholar]
- Yokota S., Deimann W., Hashimoto T., Fahimi H. D. Immunocytochemical localization of two peroxisomal enzymes of lipid beta-oxidation in specific granules of rat eosinophils. Histochemistry. 1983;78(4):425–433. doi: 10.1007/BF00496194. [DOI] [PubMed] [Google Scholar]