Abstract
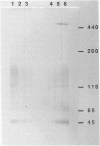
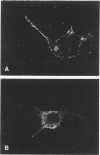
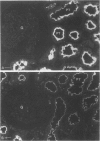
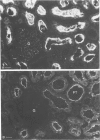
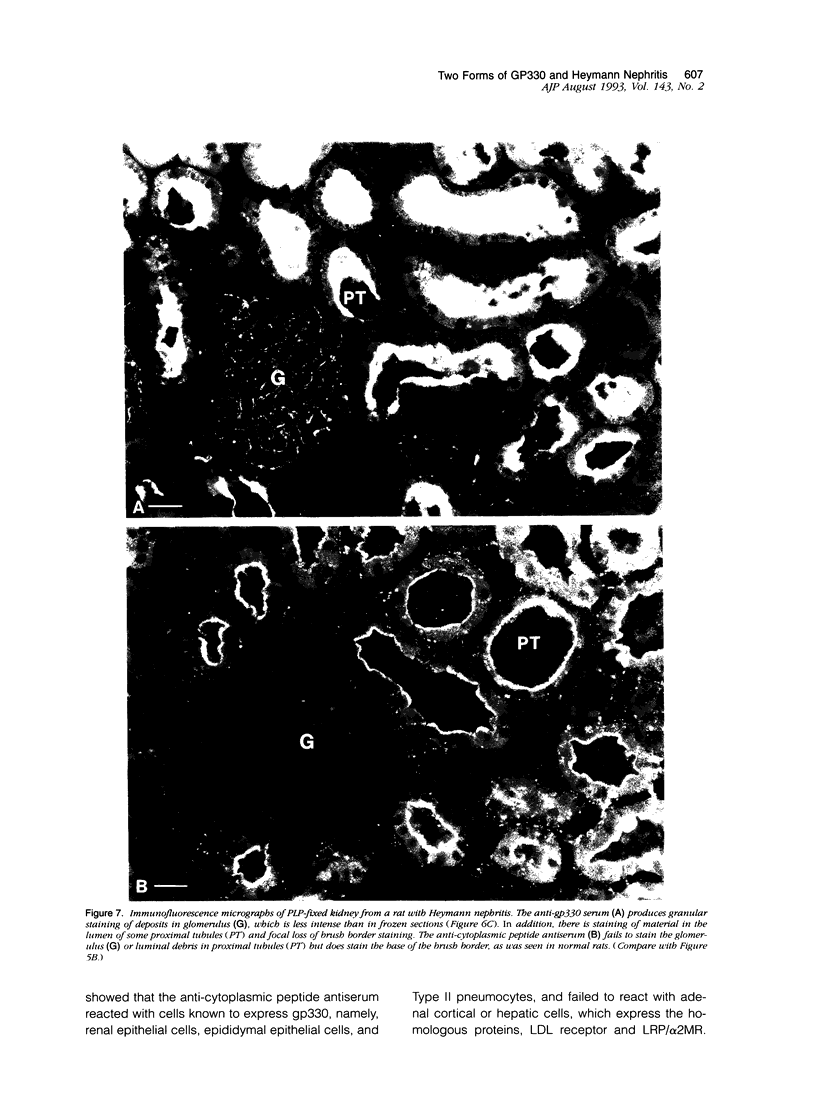
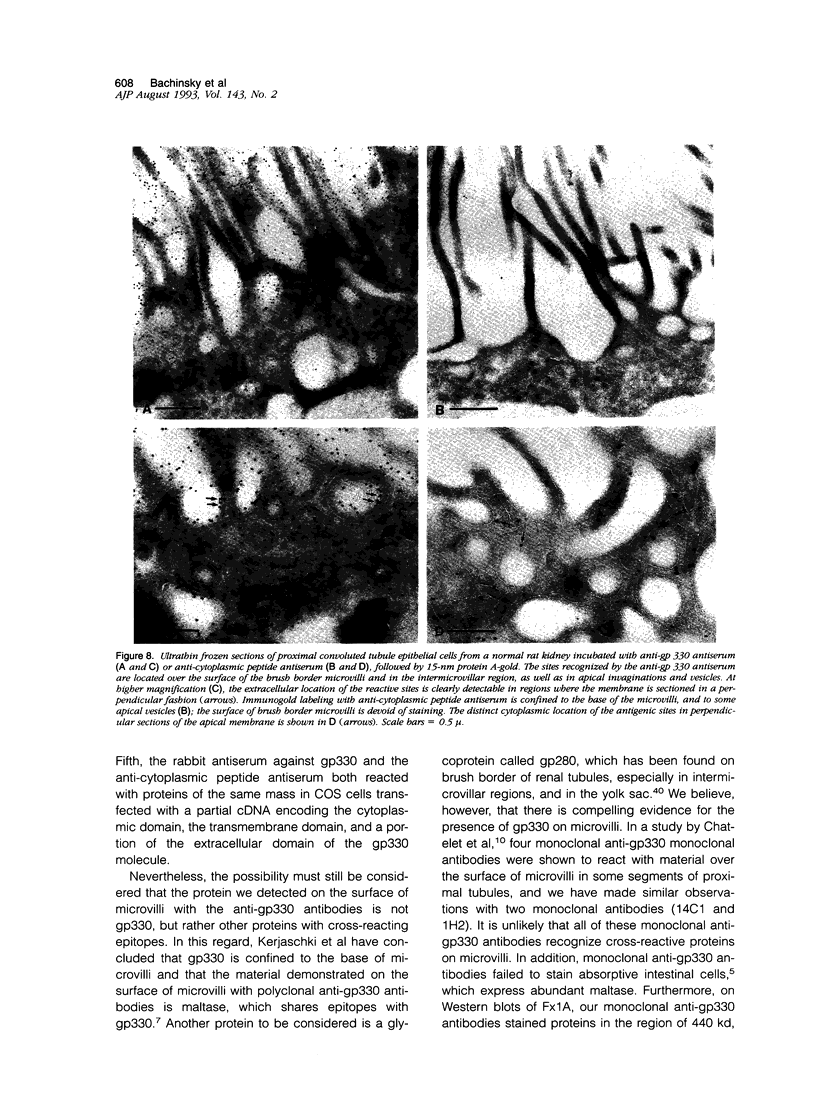
Heymann nephritis is characterized by glomerular immune deposits that contain a glycoprotein called gp330. The deposits are believed to result from shedding of immune complexes formed on podocytes. Complexes are also shed from proximal tubule cells, when antibodies combine with gp330 on the cell surface. We performed the present study to investigate what portion of the gp330 molecule is shed, using a rabbit antiserum against a peptide deduced to be in the cytoplasmic domain of gp330, as well as a rabbit antiserum and two monoclonal antibodies that recognize extracellular epitopes of gp330. The anti-cytoplasmic peptide antiserum precipitated from Fx1A (a crude renal cortical membrane preparation), a protein with a mass of about 440 kd that was reactive with two monoclonal anti-gp330 antibodies. (In our experiments, the protein called gp330 generally has a mass estimated to be about 440 kd.) The anti-cytoplasmic peptide antiserum also reacted with a truncated gp330 protein produced in transfected COS cells. Immunohistochemical studies showed that all the antibodies recognized the same group of epithelial cells. However, as seen in immunoultrastructural studies of proximal tubules, the anti-cytoplasmic peptide antiserum reacted only with components at the base of microvilli, whereas the anti-gp330 ectodomain antibodies identified material not only at the base, but over the surface of microvilli as well. In rats with Heymann nephritis, glomerular deposits and material shed into tubule lumens reacted with antibodies against extracellular epitopes of gp330, but not with the anti-cytoplasmic peptide antiserum. We propose that there are two forms of gp330 on the cell surface of proximal renal tubules. One form is restricted to coated pit regions at the base of microvilli and has a cytoplasmic domain containing a sequence deduced from a partial complementary DNA encoding gp330. The other form is present over microvilli (and possibly at the base of microvilli as well) and lacks the cytoplasmic domain deduced from the complementary DNA. The complexes that are shed in Heymann nephritis contain either a portion of gp330 cleaved from the full-length molecule or a form of gp330 that lacks the cytoplasmic domain.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman L. G., Schneider B. G., Papermaster D. S. Rapid embedding of tissues in Lowicryl K4M for immunoelectron microscopy. J Histochem Cytochem. 1984 Nov;32(11):1217–1223. doi: 10.1177/32.11.6436366. [DOI] [PubMed] [Google Scholar]
- Andres G., Brentjens J. R., Caldwell P. R., Camussi G., Matsuo S. Formation of immune deposits and disease. Lab Invest. 1986 Nov;55(5):510–520. [PubMed] [Google Scholar]
- Assmann K. J., Lange W. P., Tangelder M. M., Koene R. A. The organ distribution of gp-330 (Heymann antigen) and gp-90 in the mouse and the rat. Virchows Arch A Pathol Anat Histopathol. 1986;408(5):541–553. doi: 10.1007/BF00705307. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., Schneeberger E. E., Baird L. G., Collins A. B., Kamata K., Bradford D., Erikson M. E., McCluskey R. T. Studies with monoclonal antibodies against brush border antigens in Heymann nephritis. Lab Invest. 1985 Oct;53(4):421–432. [PubMed] [Google Scholar]
- Biemesderfer D., Dekan G., Aronson P. S., Farquhar M. G. Assembly of distinctive coated pit and microvillar microdomains in the renal brush border. Am J Physiol. 1992 Jan;262(1 Pt 2):F55–F67. doi: 10.1152/ajprenal.1992.262.1.F55. [DOI] [PubMed] [Google Scholar]
- Camussi G., Brentjens J. R., Noble B., Kerjaschki D., Malavasi F., Roholt O. A., Farquhar M. G., Andres G. Antibody-induced redistribution of Heymann antigen on the surface of cultured glomerular visceral epithelial cells: possible role in the pathogenesis of Heymann glomerulonephritis. J Immunol. 1985 Oct;135(4):2409–2416. [PubMed] [Google Scholar]
- Chatelet F., Brianti E., Ronco P., Roland J., Verroust P. Ultrastructural localization by monoclonal antibodies of brush border antigens expressed by glomeruli. I. Renal distribution. Am J Pathol. 1986 Mar;122(3):500–511. [PMC free article] [PubMed] [Google Scholar]
- Chatelet F., Brianti E., Ronco P., Roland J., Verroust P. Ultrastructural localization by monoclonal antibodies of brush border antigens expressed by glomeruli. II. Extrarenal distribution. Am J Pathol. 1986 Mar;122(3):512–519. [PMC free article] [PubMed] [Google Scholar]
- Gutmann E. J., Niles J. L., McCluskey R. T., Brown D. Colchicine-induced redistribution of an apical membrane glycoprotein (gp330) in proximal tubules. Am J Physiol. 1989 Aug;257(2 Pt 1):C397–C407. doi: 10.1152/ajpcell.1989.257.2.C397. [DOI] [PubMed] [Google Scholar]
- Gutmann E. J., Niles J. L., McCluskey R. T., Brown D. Loss of antigens associated with the apical endocytotic pathway in proximal tubules from rats with heymann nephritis. Am J Pathol. 1991 May;138(5):1243–1255. [PMC free article] [PubMed] [Google Scholar]
- HEYMANN W., HACKEL D. B., HARWOOD S., WILSON S. G., HUNTER J. L. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med. 1959 Apr;100(4):660–664. doi: 10.3181/00379727-100-24736. [DOI] [PubMed] [Google Scholar]
- Herz J., Goldstein J. L., Strickland D. K., Ho Y. K., Brown M. S. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol Chem. 1991 Nov 5;266(31):21232–21238. [PubMed] [Google Scholar]
- Herz J., Hamann U., Rogne S., Myklebost O., Gausepohl H., Stanley K. K. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988 Dec 20;7(13):4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J., Kowal R. C., Goldstein J. L., Brown M. S. Proteolytic processing of the 600 kd low density lipoprotein receptor-related protein (LRP) occurs in a trans-Golgi compartment. EMBO J. 1990 Jun;9(6):1769–1776. doi: 10.1002/j.1460-2075.1990.tb08301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata K., Baird L. G., Erikson M. E., Collins A. B., McCluskey R. T. Characterization of antigens and antibody specificities involved in Heymann nephritis. J Immunol. 1985 Oct;135(4):2400–2408. [PubMed] [Google Scholar]
- Kanalas J. J., Makker S. P. Identification of the rat Heymann nephritis autoantigen (GP330) as a receptor site for plasminogen. J Biol Chem. 1991 Jun 15;266(17):10825–10829. [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983 Feb 1;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5557–5561. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Miettinen A., Farquhar M. G. Initial events in the formation of immune deposits in passive Heymann nephritis. gp330-anti-gp330 immune complexes form in epithelial coated pits and rapidly become attached to the glomerular basement membrane. J Exp Med. 1987 Jul 1;166(1):109–128. doi: 10.1084/jem.166.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Noronha-Blob L., Sacktor B., Farquhar M. G. Microdomains of distinctive glycoprotein composition in the kidney proximal tubule brush border. J Cell Biol. 1984 Apr;98(4):1505–1513. doi: 10.1083/jcb.98.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen J., Sugisaki T., Milgrom F., McCluskey R. T. Studies on multiple renal lesions in Heymann nephritis. Lab Invest. 1971 Dec;25(6):577–585. [PubMed] [Google Scholar]
- Kounnas M. Z., Argraves W. S., Strickland D. K. The 39-kDa receptor-associated protein interacts with two members of the low density lipoprotein receptor family, alpha 2-macroglobulin receptor and glycoprotein 330. J Biol Chem. 1992 Oct 15;267(29):21162–21166. [PubMed] [Google Scholar]
- Kristensen T., Moestrup S. K., Gliemann J., Bendtsen L., Sand O., Sottrup-Jensen L. Evidence that the newly cloned low-density-lipoprotein receptor related protein (LRP) is the alpha 2-macroglobulin receptor. FEBS Lett. 1990 Dec 10;276(1-2):151–155. doi: 10.1016/0014-5793(90)80530-v. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mendrick D. L., Chung D. C., Rennke H. G. Heymann antigen GP330 demonstrates affinity for fibronectin, laminin, and type I collagen and mediates rat proximal tubule epithelial cell adherence to such matrices in vitro. Exp Cell Res. 1990 May;188(1):23–35. doi: 10.1016/0014-4827(90)90273-d. [DOI] [PubMed] [Google Scholar]
- Moestrup S. K., Gliemann J. Purification of the rat hepatic alpha 2-macroglobulin receptor as an approximately 440-kDa single chain protein. J Biol Chem. 1989 Sep 15;264(26):15574–15577. [PubMed] [Google Scholar]
- Niles J. L., McCluskey R. T., Ahmad M. F., Arnaout M. A. Wegener's granulomatosis autoantigen is a novel neutrophil serine proteinase. Blood. 1989 Nov 1;74(6):1888–1893. [PubMed] [Google Scholar]
- Orlando R. A., Kerjaschki D., Kurihara H., Biemesderfer D., Farquhar M. G. gp330 associates with a 44-kDa protein in the rat kidney to form the Heymann nephritis antigenic complex. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6698–6702. doi: 10.1073/pnas.89.15.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietromonaco S., Kerjaschki D., Binder S., Ullrich R., Farquhar M. G. Molecular cloning of a cDNA encoding a major pathogenic domain of the Heymann nephritis antigen gp330. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1811–1815. doi: 10.1073/pnas.87.5.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychowdhury R., Niles J. L., McCluskey R. T., Smith J. A. Autoimmune target in Heymann nephritis is a glycoprotein with homology to the LDL receptor. Science. 1989 Jun 9;244(4909):1163–1165. doi: 10.1126/science.2786251. [DOI] [PubMed] [Google Scholar]
- Sahali D., Mulliez N., Chatelet F., Laurent-Winter C., Citadelle D., Roux C., Ronco P., Verroust P. Coexpression in humans by kidney and fetal envelopes of a 280 kDa-coated pit-restricted protein. Similarity with the murine target of teratogenic antibodies. Am J Pathol. 1992 Jan;140(1):33–44. [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Strickland D. K., Ashcom J. D., Williams S., Burgess W. H., Migliorini M., Argraves W. S. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990 Oct 15;265(29):17401–17404. [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]