Abstract
We previously reported that treatment and withdrawal from cocaine increases: (1) serotonin 2A (5-HT2A) receptor-mediated neuroendocrine responses, and (2) Gαq and Gα11 G-protein levels in the hypothalamic paraventricular nucleus (PVN) at 48 h post-treatment. This study investigates changes in the initial 24 h of withdrawal to discern whether 5-HT2A receptor supersensitivity is due to cocaine treatment or is induced during the withdrawal period. We report here increases in 5-HT2A receptor-mediated neuroendocrine responses only 12 or 24 h post-treatment, but not during the initial 4 h withdrawal period. Levels of membrane- or cytosol-associated Gαq or Gα11 proteins in PVN are not altered during the first 24 h of withdrawal. However, the density of 125I-DOI-labeled high-affinity 5-HT2A receptors in PVN increased 35% in rats withdrawn from cocaine for 24 h. These findings demonstrate that cocaine-induced increases in 5-HT2A receptor function in PVN represents a withdrawal-induced phenomena that: (1) is likely attributed to increased G-protein coupled/high-affinity conformational state of the 5-HT2A receptor, and (2) occurs in the absence of changes in the levels of associated G proteins during the first 24 h.
Keywords: serotonin, hypothalamus, prolactin, corticosterone, G proteins, rats
INTRODUCTION
The activity of the hypothalamic–pituitary–adrenal (HPA) axis before, during and after cocaine exposure appears to be one of the important determinants of whether or not individuals will engage in cocaine-seeking behavior (Sinha et al., 2006; Fox et al., 2005; Mantsch et al., 2003; Sinha et al., 2000; Sinha et al., 2003). While chronic cocaine administration is associated with neuroadpative changes that lead to attenuated responses to cocaine in humans and rats (Levy et al., 1993; Mendelson et al., 1998), withdrawal from cocaine is associated with activation of the HPA axis (Peltier et al., 2001; Zhou et al., 2003). This enhanced response of the HPA axis during withdrawal from chronic cocaine has been reported 24 h to 48 h after the last cocaine injection in rats (Peltier et al., 2001; Zhou et al., 2003). Similar results have been reported in human cocaine addicts (Vescovi et al., 1992; Mendelson et al., 1998).
We and others have previously reported unique neuroadaptive changes in the serotonin 2A (5-HT2A) receptor signaling pathway in the hypothalamic paraventricular nucleus (PVN) after 2 days of withdrawal from cocaine. These include: (1) increases in 5-HT2A receptor-mediated neuroendocrine responses, suggesting supersensitivity of postsynaptic 5-HT2A receptors in PVN (Levy et al., 1992; Baumann et al., 1993; Baumann and Rothman, 1998); and (2) increases in Gαq and Gα11 G-proteins in the membrane fraction of PVN (Carrasco et al., 2003; Carrasco et al., 2004b). Since agonists and antagonists of 5-HT2A receptors produces desensitization of 5-HT2A receptors (Gray and Roth, 2001; Hanley and Hensler, 2002; Damjanoska et al., 2004), the increases in 5-HT2A receptor signaling during withdrawal from cocaine represents a unique adaptive response of this system.
The objectives of the present study were to determine if changes in neuroendocrine responses to (−)DOI (a 5-HT2A/2C receptor agonist) and the levels of Gα11 and Gαq G-proteins in the PVN are produced during the cocaine treatment period or develop during withdrawal. Gαq and Gα11 proteins were examined because these are the G-proteins that mediate 5-HT2A receptor function. As 5-HT2A receptors in PVN are important for the regulation of mood, impulse control and responses to stressors (Kroeze et al., 2002; Aghajanian and Marek, 2000), withdrawal-induced increases in 5-HT2A receptor function in this limbic region may be clinically relevant with respect to drug relapse.
MATERIALS AND METHODS
Drugs
(−)Cocaine-HCl was obtained from the National Institute on Drug Abuse (Bethesda, MD). A fresh cocaine solution (15mg/ml) was prepared in 0.9% saline prior to each dosing. (−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane HCl [(−)DOI] was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in 0.9% saline to the concentration of 0.5 mg/kg/ml. All solutions were made fresh before administration and injected at a volume of 1 ml/kg.
Treatment
Male Sprague-Dawley rats (225–275 g) were purchased from Harlan (Indianapolis, IN). The rats were housed two per cage in a temperature-, humidity-, and light-controlled room (12 hr light/dark cycle, lights on 7:00 AM–19:00 PM). Food and water were available ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals as approved by Loyola University Institutional Animal Care and Use Committee (IACUC).
Experimental Protocols
After arrival, the rats were allowed to acclimate to their environment for at least 4 days prior to the start of the treatment period. Eight to twelve rats were randomly assigned to each group. Cage-mates were assigned to the same treatment group. Rats were injected with either 0.9% saline (1ml/kg, i.p., 8:30 h and 15:30 h) or cocaine (15mg/kg, i.p., 8:30 h and 15:30 h) for 5 days. At different time points after the last saline or (−)cocaine injection (0.5, 1, 2, 4, 12, and 24 hrs), rats were sacrificed by decapitation. The rats were challenged with either saline (1ml/kg) or (−)DOI (0.5 mg/kg) 30 min prior to sacrifice. The trunk blood was collected in centrifuge tubes containing a 0.5 ml solution of 0.3 M EDTA (pH 7.4). After centrifugation, the plasma was stored at −80°C for radioimmunoassays of plasma hormone concentrations. The brains were immediately removed and frozen in dry ice and stored at −80°C.
Radioimmunoassay
Plasma prolactin and corticosterone concentrations were determined by radioimmunoassays as previously described in detail (Li et al., 1993; Li et al., 1997).
Tissue preparation
Rat brains were placed in a cryostat at −12°C, and coronal sections were cut to obtain a 700 μm thick section containing the PVN. The PVN was microdissected from these frozen sections with the aid of a dissecting stereomicroscope.
Western blots
Plasma membranes and cytosol fractions of the PVN were prepared as previously reported (Carrasco et al., 2003). Expression of membrane- and cytosol-associated Gαq, Gα11 and Gαz proteins was measured by Western blot as previously described (Carrasco et al., 2004b). Negative controls included either omission of primary antibody or addition of pre-immune rabbit immunoglobulins.
Western Blot film analysis
Films were analyzed densitometrically with values calculated from the integrated optical density (IOD) of each band using Scion Image software (Scion Corporation, Frederick, MD, USA), as previously described (Carrasco et al., 2004a). Each sample was measured on three independent gels. All samples were standardized to controls and normalized to their respective actin levels.
Autoradiography of 125I-DOI binding
Only rats injected with saline before sacrifice were used for receptor autoradiography. The density of 5-HT2A receptors in PVN was examined by autoradiographic labeling with 125I-DOI (2,200 Ci/mmol) in slide mounted sections as previously described (Appel et al., 1990). Nonspecific binding was determined in the presence of 100nM spiperone to block 125I-DOI binding to 5-HT2A receptors. A set of 125I microscale standards (Amersham Biosciences, Piscataway NJ) were included with samples to equate film optical density readings to fmol/mg of tissue equivalent based on the specific activity of the radioligand. All slides were apposed to Kodak Bio-Max MR film for 6 days at 4°C. Images were scanned and analyzed using MCID Elite 7.0 software (Imaging Research, St-Catharines, Ontario, Canada). Specific 125I-DOI binding was determined by subtracting the nonspecific binding from the total binding. Data for each rat are the mean of five adjacent sections.
Statistics
All data are expressed as the mean ± S.E.M., where n indicates the number of rats per group. Hormone data were analyzed by three-way analyses of variance (ANOVA). Immunoblot data was analyzed by two-way ANOVA. Group means were compared by a Newman-Keuls multiple-range test (Steel and Torrie, 1960). Autoradiography data was analyzed by an unpaired Student’s t-test. GB-STAT software (Dynamic Microsystems, Inc., Silver Spring, MD, USA) was used for all statistical analyses.
RESULTS
Cocaine withdrawal associated increases in (−)DOI-induced hormone levels
Our previous experiments did not discern whether changes in the activity of 5-HT2A receptors in the PVN after treatment and withdrawal from cocaine were a cocaine-treatment effect or a withdrawal-induced phenomena. Therefore, we investigated the neuroendocrine responses to (−)DOI in rats injected for 5 days with either saline or cocaine and withdrawn for 0.5 to 24 h. Increased sensitivity of 5-HT2A receptors that stimulate the secretion of corticosterone and prolactin was observed only following 12 or 24 h withdrawal from repeated cocaine treatment (Fig. 1A and 1B). No supersensitivity occurred within the first 1–4 h of cocaine withdrawal. As shown in Fig. 1, basal corticosterone and prolactin levels were similar (p>0.05) in saline and cocaine treated rats at all post-treatment withdrawal times. (−)DOI significantly increased the plasma levels of corticosterone and prolactin in saline- or cocaine-treated rats at all post-treatment times (Fig. 1). However, in cocaine-treated rats, the (−)DOI stimulated increase in corticosterone and prolactin was significantly greater only at 12 and 24 h withdrawal times, indicating that 5-HT2A receptor supersensitivity is a result of changes occurring specifically during the withdrawal period.
Figure 1.
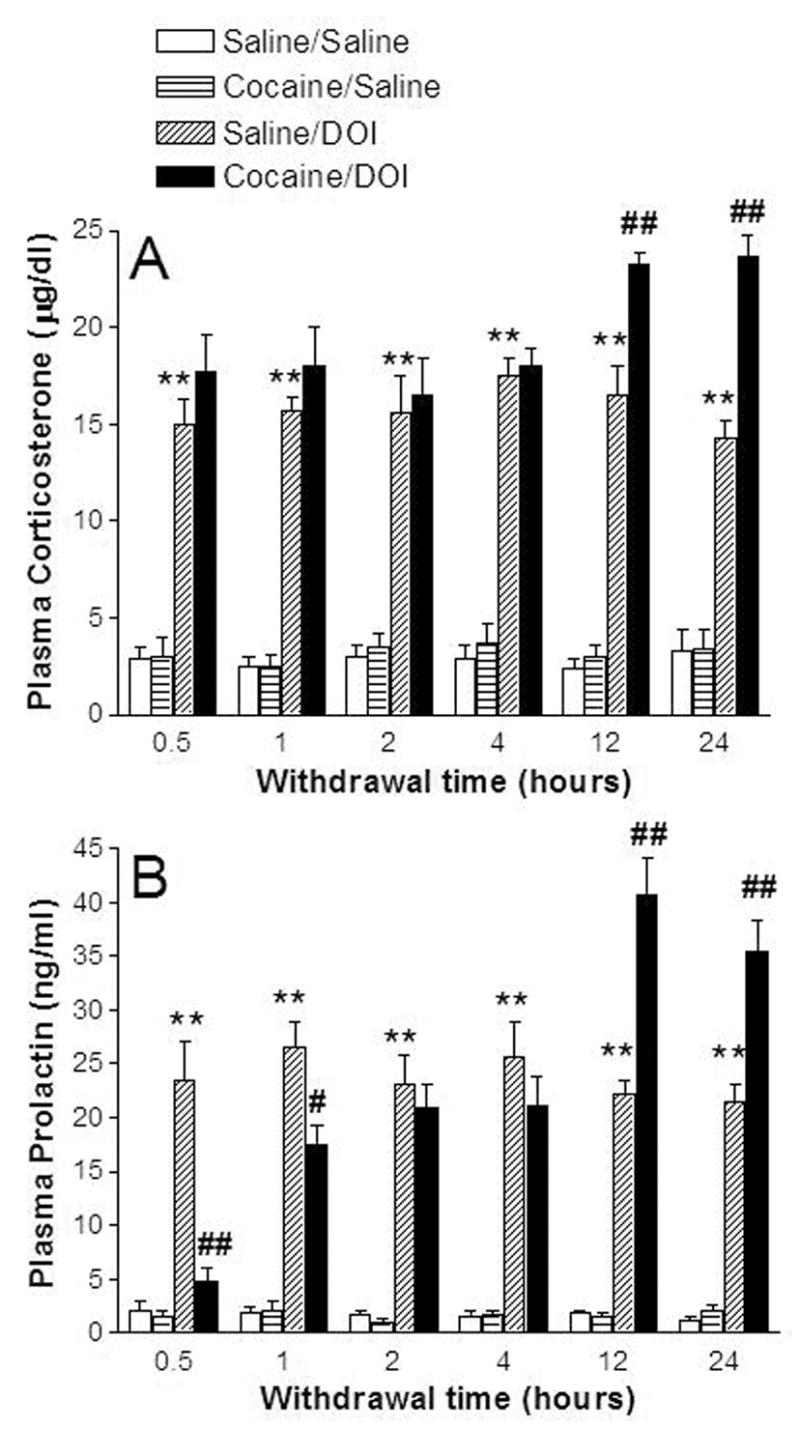
Time-dependent supersensitivity of the (−)DOI-induced neuroendocrine responses in cocaine-treated rats at various post-treatment withdrawal times. The data represent the plasma levels of corticosterone (A) and prolactin (B) in rats treated chronically with saline (1ml/kg, i.p., bid) or cocaine (15mg/kg, i.p., bid) and challenged with saline (1 ml/kg) or (−)DOI (0.5 mg/kg) (mean ± SEM of 8–12 rats per group). **p <0.01, significant effect of (−)DOI compared with the respective saline challenge group. ##p < 0.01, significant effect of (−)DOI challenge in cocaine-treated rats compared with (−)DOI challenge in saline-treated rats (three-way ANOVA and Newman-Keuls multiple range test).
For plasma corticosterone, the three-way ANOVA indicated a significant main effect of treatment (F(1,140)= 26.25, p<0.0001) and a significant main effect of (−)DOI challenge (F(1,140)= 922.23, p<0.0001), but not a main effect of withdrawal time (F(1,140)= 3.53, p<0.88). Significant interactions between treatment and the (−)DOI challenge (F(1,140)= 12.25, p<0.0006), and between treatment, withdrawal time and (−)DOI challenge (F(5,140)= 2.39, p<0.0407) were found. The post hoc Newman-Keuls test indicated no differences in the (−)DOI-induced plasma levels of corticosterone between cocaine- and saline-treated rats at any of the times during the first four hours of withdrawal. However, (−)DOI-induced plasma corticosterone levels in cocaine-treated rats were significantly (p<0.01) higher than in saline-treated controls at 12 and 24 h of withdrawal from cocaine (Fig. 1A). The magnitude of increase in (−)DOI-induced corticosterone responses were similar (45% increase) at 12 and 24 h of withdrawal from cocaine (Fig. 1A).
For plasma prolactin, the three-way ANOVA indicated a significant main effect of withdrawal time (F(5,158)= 12.02, p<0.0001) and (−)DOI challenge (F(1,158)= 924.52, p<0.0001) but not a main effect of treatment (F(1,158)= 4.79, p<0.73). There was a significant interaction between treatment and withdrawal time (F(5,158)= 20.12, p<0.00001), and between withdrawal time and (−)DOI challenge (F(5,158)= 12.94, p<0.0001). There was also a significant interaction between cocaine treatment, withdrawal time and (−)DOI challenge (F(5,158)= 18.03, p<0.0001). At 30 and 60 min of withdrawal the plasma levels of prolactin are lower in cocaine-treated rats than in saline-treated rats (p<0.01 and p<0.05, respectively) (Fig. 1B). The post hoc Newman-Keuls test indicated that (−)DOI-induced plasma prolactin levels in cocaine-treated rats are significantly (p<0.01) higher than the (−)DOI-induced increases in saline treated rats at 12 or 24 h of withdrawal (Fig. 1B). The magnitude of increases in (−)DOI-induced prolactin levels were similar (65% increase) at 12 and 24 h of withdrawal from cocaine.
Membrane levels of Gα proteins
Gαq protein was detected in PVN at approximately 42–43 kDa (Fig. 2A). The levels of membrane-associated Gαq in the PVN were not affected by cocaine treatment (F(1,71)= 0.70536, p> 0.41) or withdrawal period (F(5,71)= 0.2532, p> 0.94) (Fig. 2B). Neither cocaine treatment nor withdrawal time had a significant (p >0.05) effect on the levels of cytosol-associated Gαq in PVN (Fig. 2A).
Figure 2.
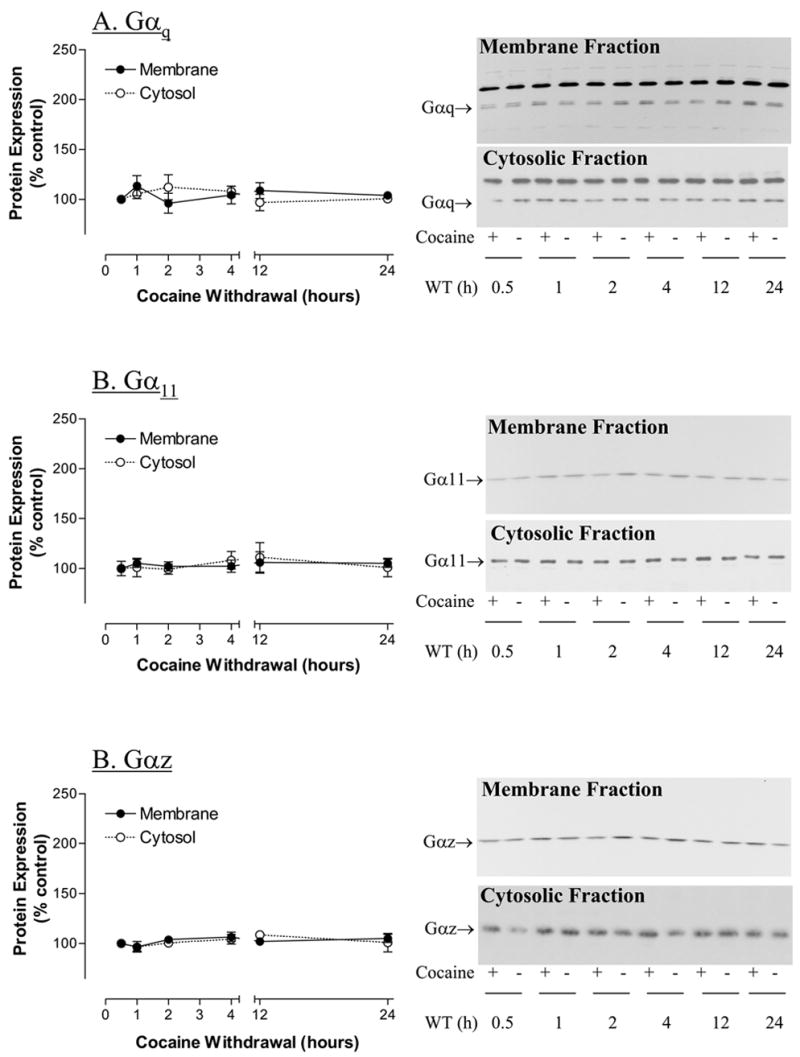
The effect of withdrawal time on the levels of membrane- and cytosol-associated protein levels of (A) Gαq proteins; (B) Gα11 proteins; and (C) Gαz proteins in hypothalamic paraventricular nucleus of rats treated with saline or cocaine (15 mg/kg, ip, 5 days). WT= withdrawal time in hours (h). β-Actin was used as control of protein loading (data not shown). The data represent mean IOD (Integrated Optical Density) ± SEM as % control (n=6–8) measured in triplicate.
Gα11 protein was detected in PVN at approximately 40 kDa (Fig. 2B). There was no significant main effect of cocaine treatment on the levels of membrane-associated Gα11 protein in the PVN (F(1,71)= 0.3035, p<0.5837). Similarly, there was no significant main effect of withdrawal time on the levels of membrane-associated Gα11 protein in the PVN (F(5,71)= 0.2434, p<0.9415). Neither cocaine treatment nor withdrawal time had a significant (p >0.05) effect on the levels of cytosol-associated Gα11 in PVN (Fig. 2B).
Gαz is a 40 kDa protein that has not been reported to be associated with the 5-HT2A receptor signaling cascade. The determination of Gαz protein was used to verify the specificity of the effects of cocaine treatment on G-proteins associated with 5-HT2 receptor signal transduction. There was no significant main effect of cocaine on the levels of membrane-associated Gαz in PVN (F(1,71)= 0.1726, p<0.6792) (Fig. 2C). There was no significant effect of withdrawal time on the levels of membrane-associated Gαz in PVN (F(5,71)= 0.09458, p<0.9927). The levels of cytosol-associated Gαz in PVN were not affected (p>0.05) by cocaine treatment or withdrawal time (Fig. 2C).
Autoradiography of 5-HT2A Receptors
Figure 3A shows representative brightfield autoradiograms of the distribution of 125I-DOI binding in coronal sections of rats at 24 h withdrawal from five days treatment with either saline or cocaine. The insert is a magnified view of the paraventricular nucleus of the hypothalamus in the respective sections. As shown in Fig. 3B, the density of 125I-DOI-labeled 5-HT2A receptors in PVN was significantly (p<0.05) increased (+35%) in rats at 24 hours withdrawal from cocaine.
Figure 3.
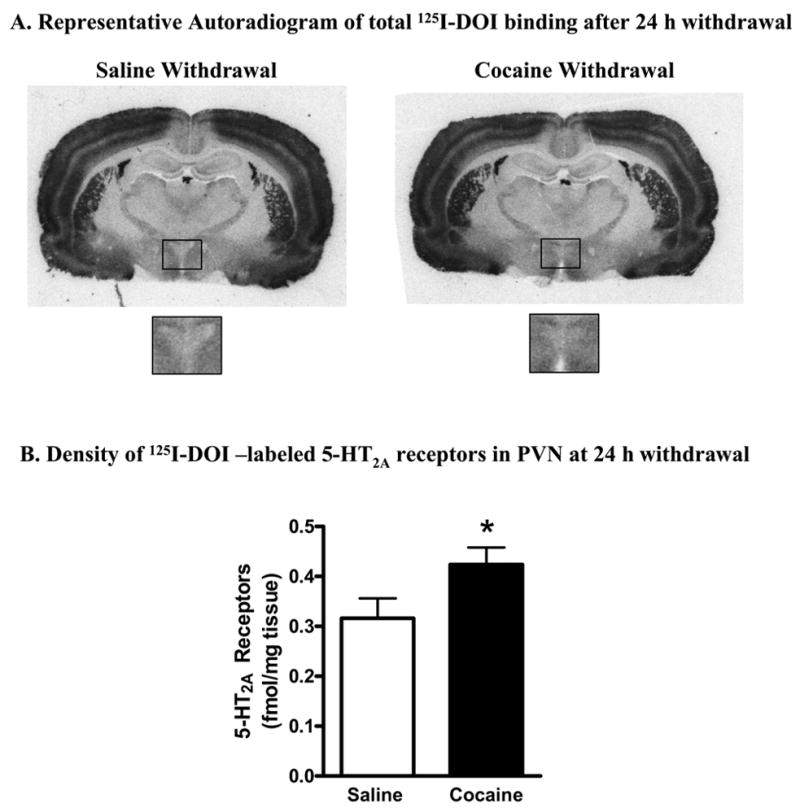
125I-DOI-labeled 5-HT2A receptors in hypothalamic paraventricular nucleus of rats withdrawn from cocaine for 24 h. (A) Representative autoradiogram of 125I-DOI binding performed in coronal sections from rats withdrawn for 24 h from saline or cocaine treatment. The box on this coronal section indicates the region of the paraventricular nucleus of the hypothalamus. Higher power images of the paraventricular nucleus of the hypothalamus are shown for a representative saline- and cocaine-treated rats. (B) Quantification of 125I-DOI labeled 5-HT2A receptors in PVN of rats withdrawn from either saline or cocaine for 24 h. 125I-DOI-labeled 5-HT2A receptors were significantly increased in rats withdrawn from cocaine for 24 h. The data represent mean ± SEM of 5 rats per group. * p<0.05 between cocaine- and saline- treated rats (unpaired Student-t test).
Discussion
The present findings demonstrate that 5-HT2A receptor supersensitivity in PVN is not present immediately following cocaine treatment but develops during the early withdrawal period. More specifically, cocaine-treated rats required at least 4 h of withdrawal to develop increases in (−)DOI-induced secretion of corticosterone and prolactin, since changes were first observed at 12 h post-treatment. The supersensitivity of 5-HT2A receptors in the PVN is associated with increases in the density of the G-protein coupled/high-affinity conformational state of 5-HT2A receptors at 24h withdrawal but not with increased levels of membrane- or cytosol-associated Gαq and Gα11 proteins, as these were not altered during the first 24 h of withdrawal.
It is well known that chronic treatment with agonists or antagonists of 5-HT2A receptors typically produces desensitization of 5-HT2A receptors (Roth et al., 1998; Gray and Roth, 2001; Hanley and Hensler, 2002; Damjanoska et al., 2004). In contrast, withdrawal from cocaine produces supersensitivity of 5-HT2A receptors (Carrasco et al., 2003; Carrasco et al., 2004b). In the present study, the (−)DOI-evoked release of corticosterone and prolactin was greater in rats undergoing cocaine withdrawal, a result that is consistent with reports in the literature (Baumann et al., 1993; Baumann and Rothman, 1998; Levy et al., 1992). We have previously reported that the neuroendocrine effects of (−)DOI, are mediated exclusively by activation of 5-HT2A receptors (but not 5-HT2C receptors) in the PVN (Zhang et al., 2002). Consequently, the potentiated responses to (−)DOI of two different hormones (prolactin and corticosterone) that are mediated by distinct signaling pathways suggests supersensitivity mediated at the level of 5-HT2A receptor signal transduction.
In contrast to the increased 5-HT2A receptor-mediated prolactin response at 12 and 24 h post-treatment, the prolactin response to (−)DOI was blunted after 0.5 and 1 h of withdrawal from cocaine. Increased dopamine levels, due to cocaine blockade of dopamine re-uptake and D2 dopaminergic inhibition of prolactin release, could explain this blunted response to (−)DOI at 0.5 and 1 h post-treatment, when cocaine is most likely to still be present (Samson et al., 2003). As no increases in 5-HT2A receptor-mediated corticosterone levels were observed at 0.5 and 1 h post-treatment times, these data provide further evidence that 5-HT2A receptor supersensitivity is a result of changes occurring during the withdrawal period.
Behavioral and neuroendocrine studies in rats withdrawn from cocaine indicate no increase in the maximal responses to (−)DOI, but do reveal a left-ward-shift in the dose-response curves (Baumann et al., 1993; Baumann and Rothman, 1998; Levy et al., 1992). These data suggest that 5-HT2A receptor supersensitivity could be the result of alterations in receptor-G-protein coupling or alterations in G-protein activation of signal transduction mechanisms. Our present results support this interpretation, as we detected an increase in 125I-DOI-labeled 5-HT2A receptors in rats withdrawn from cocaine for 24 h in the absence of increases in the levels of membrane-associated Gαq and Gα11 proteins in the PVN. Importantly, we have reported increases in the levels of membrane-associated Gαq and Gα11 proteins in the PVN that require at least 48 h of withdrawal (Carrasco et al., 2003; Carrasco et al., 2004b), suggesting longer withdrawal times may evoke additional mechanism(s) of supersensitivity of 5-HT2A receptor-mediated responses.
In summary, our results demonstrate that supersensitivity of 5-HT2A receptors in the PVN is a result of changes occurring specifically during the withdrawal period that requires at least 4 h to develop. Although, the mechanisms by which cocaine withdrawal mediates 5-HT2A receptor supersensitivity in PVN are not completely understood, our present and published data indicates that they could involve: (1) increased density of 5-HT2A receptors in a “high-affinity” state at least at 24 h of withdrawal; and (2) increased levels of membrane-associated Gαq and Gα11 proteins at 48 h of withdrawal. As 5-HT2A receptors in PVN are important for the regulation of mood, impulse control and responses to stressors (Kroeze et al., 2002; Aghajanian and Marek, 2000), withdrawal-induced increases in 5-HT2A receptor function in this limbic region may be clinically relevant with respect to drug relapse.
Acknowledgments
This work was supported in part by DA13669 and NS34153.
Abbreviations
- (HPA) axis
Hypothalamic–Pituitary–Adrenal
- (PVN)
Hypothalamic Paraventricular Nucleus
- (5-HT)
5-hydroxytryptamine
- (5-HT2A receptors)
Serotonin 2A receptors
- HCL (DOI)
(−)-1-(2,5 dimethoxy-4-iodophenyl)-2-amino-propane
Footnotes
Section Editor: Dr. Yoland Smith, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road NE, Atlanta, GA 30329, USA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Rev. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Appel NM, Mitchell WM, Garlick RK, Glennon RA, Teiteler M, de Souza EB. Autoradiographic characterization of (±)-1-(2,5-dimethoxy- 4- [125I]iodophenyl)-2-aminopropane ([125I]DOI) binding to 5- HT2 and 5-HT1c receptors in rat brain. J Pharmacol Exp Ther. 1990;255:843–857. [PubMed] [Google Scholar]
- Baumann MH, Brockington AM, Rothman RB. Withdrawal from chronic cocaine enhances behavioral sensitivity to the 5-HT2/1C agonist DOI. Biol Psychiatry. 1993;34:576–577. doi: 10.1016/0006-3223(93)90204-q. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Rothman RB. Alterations in serotonergic responsiveness during cocaine withdrawal in rats: Similarities to major depression in humans. Biol Psychiatry. 1998;44:578–591. doi: 10.1016/s0006-3223(98)00123-1. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Barker SA, Zhang Y, Damjanoska KJ, Sullivan NR, Garcia F, D'Souza DN, Muma NA, Van de Kar LD. Estrogen treatment increases the levels of regulator of G protein signaling-Z1 in the hypothalamic paraventricular nucleus: possible role in desensitization of 5-hydroxytryptamine(1A) receptors. Neuroscience. 2004a;127:261–267. doi: 10.1016/j.neuroscience.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Damjanoska KJ, D'Souza DN, Zhang Y, Garcia F, Battaglia G, Muma NA, Van de Kar LD. Short-Term Cocaine Treatment Causes Neuroadaptive Changes in G{alpha}q and G{alpha}11 Proteins in Rats Undergoing Withdrawal. J Pharmacol Exp Ther. 2004b;311:349–355. doi: 10.1124/jpet.104.069807. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Zhang Y, Damjanoska KJ, D'Souza DN, Garcia F, Battaglia G, Muma NA, Van de Kar LD. A region-specific increase inGαq and Gα11 proteins in brains of rats during cocaine withdrawal. J Pharmacol Exp Ther. 2003;307:1012–1019. doi: 10.1124/jpet.103.056978. [DOI] [PubMed] [Google Scholar]
- Damjanoska KJ, Heidenreich BA, Kindel GH, D'Souza DN, Zhang Y, Garcia F, Battaglia G, Wolf WA, Van de Kar LD, Muma NA. Agonist-Induced Serotonin 2A Receptor Desensitization in the Rat Frontal Cortex and Hypothalamus. J Pharmacol Exp Ther. 2004;309:1043–1050. doi: 10.1124/jpet.103.062067. [DOI] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology. 2005;30:880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Paradoxical trafficking and regulation of 5HT2A receptors by agonists and antagonists. Brain Research Bulletin. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- Hanley NR, Hensler JG. Mechanisms of ligand-induced desensitization of the 5-hydroxytryptamine(2A) receptor. J Pharmacol Exp Ther. 2002;300:468–477. doi: 10.1124/jpet.300.2.468. [DOI] [PubMed] [Google Scholar]
- Kroeze WK, Kristiansen K, Roth BL. Molecular biology of serotonin receptors structure and function at the molecular level. Curr Top Med Chem. 2002;2:507–528. doi: 10.2174/1568026023393796. [DOI] [PubMed] [Google Scholar]
- Levy AD, Li Q, Alvarez Sanz MC, Rittenhouse PA, Brownfield MS, Van de Kar LD. Repeated cocaine modifies the neuroendocrine responses to the 5-HT1C/5-HT2 receptor agonist DOI. Eur J Pharmacol. 1992;221:121–127. doi: 10.1016/0014-2999(92)90780-8. [DOI] [PubMed] [Google Scholar]
- Levy AD, Rittenhouse PA, Bonadonna AM, Alvarez Sanz MC, Bethea CL, Van de Kar LD. Repeated exposure to cocaine produces long-lasting deficits in the serotonergic stimulation of prolactin and renin, but not adrenocorticotropin secretion. Eur J Pharmacol. 1993;241:275–278. doi: 10.1016/0014-2999(93)90215-4. [DOI] [PubMed] [Google Scholar]
- Li Q, Brownfield MS, Battaglia G, Cabrera TM, Levy AD, Rittenhouse PA, Van de Kar LD. Long-term treatment with the antidepressants fluoxetine and desipramine potentiates endocrine responses to the serotonin agonists 6-chloro-2-[1-piperazinyl]-pyrazine (MK-212) and (±)-1- (2,5-dimethoxy-4-iodophenyl)-2-aminopropane HCI (DOI) J Pharmacol Exp Ther. 1993;266:836–844. [PubMed] [Google Scholar]
- Li Q, Muma NA, Battaglia G, Van de Kar LD. A desensitization of hypothalamic 5-HT1A receptors by repeated injections of paroxetine: reduction in the levels of Gi and Go proteins and neuroendocrine responses, but not in the density of 5-HT1A receptors. J Pharmacol Exp Ther. 1997;282:1581–1590. [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Neuroendocrine alterations in a high-dose, extended-access rat self-administration model of escalating cocaine use. Psychoneuroendocrinology. 2003;28:836–862. doi: 10.1016/s0306-4530(02)00088-4. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar M, Mello NK, Teoh SK, Sholar JW. Cocaine tolerance: Behavioral, cardiovascular, and neuroendocrine function in men. Neuropsychopharmacology. 1998;18:263–271. doi: 10.1016/S0893-133X(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Peltier RL, Guerin GF, Dorairaj N, Goeders NE. Effects of saline substitution on responding and plasma corticosterone in rats trained to self-administer different doses of cocaine. J Pharmacol Exp Ther. 2001;299:114–120. [PubMed] [Google Scholar]
- Roth BL, Berry SA, Kroeze WK, Willins DL, Kristiansen K. Serotonin 5-HT2A receptors: molecular biology and mechanisms of regulation. Crit Rev Neurobiol. 1998;12:319–338. doi: 10.1615/critrevneurobiol.v12.i4.30. [DOI] [PubMed] [Google Scholar]
- Samson WK, Taylor MM, Baker JR. Prolactin-releasing peptides. Regul Pept. 2003;114:1–5. doi: 10.1016/s0167-0115(03)00110-1. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Steel RGD, Torrie JH. Principles and procedures of statistics with special reference to the biological sciences. McGraw-Hill; New York: 1960. [Google Scholar]
- Vescovi PP, Coiro V, Volpi R, Passeri M. Diurnal variations in plasma ACTH, cortisol and beta-endorphin levels in cocaine addicts. Horm Res. 1992;37:221–224. doi: 10.1159/000182316. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Damjanoska KJ, Carrasco GA, Dudas B, D'Souza DN, Tetzlaff J, Garcia F, Hanley NR, Scripathirathan K, Petersen BR, Gray TS, Battaglia G, Muma NA, Van de Kar LD. Evidence that 5-HT2A receptors in the hypothalamic paraventricular nucleus mediate neuroendocrine responses to (−)DOI. J Neurosci. 2002;22:9635–9642. doi: 10.1523/JNEUROSCI.22-21-09635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Schlussman SD, Ho A, Kreek MJ. Alterations in hypothalamic-pituitary-adrenal axis activity and in levels of proopiomelanocortin and corticotropin-releasing hormone-receptor 1 mRNAs in the pituitary and hypothalamus of the rat during chronic 'binge' cocaine and withdrawal. Brain Res. 2003;964:187–199. doi: 10.1016/s0006-8993(02)03929-x. [DOI] [PubMed] [Google Scholar]


