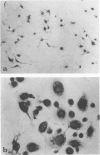
Abstract
Central nervous system disease is a frequent finding in both pediatric and adult AIDS. Microglia have been shown to be the major target of HIV-1 infection in the central nervous system. However, studies in vitro concerning susceptibility of human microglia to HIV-1 infection reported conflicting results; microglia from adult brain showed productive infection by HIV-1, whereas microglia from fetal brain did not. To investigate this further and to define the possible mechanisms responsible for this difference, we prepared highly purified human microglial cell cultures from fetuses of 16 to 24 weeks' gestation and exposed them to monocytotropic (HIV-1 JR-FL and HIV-1 JR-CSF) isolates of HIV-1. Culture supernatants were examined for the presence of p24 antigen for a 4-week period after viral exposure. Concurrently, potential cytopathic effects and cellular viral antigen expression (gp41 and p24) were examined by light microscopy in combination with immunocytochemistry. The results showed that human fetal microglia can be productively infected by HIV-1 as judged by p24 antigen capture assay, syncytia formation, and gp41 and p24 immunoreactivity of infected microglia. In addition, by electron microscopy, numerous viral particles characteristic of HIV-1 were present both in the intracellular and extracellular compartments. Uninfected cultures or astrocytes overgrown in the microglial cultures did not show evidence of infection under identical experimental conditions. These data demonstrate that human fetal microglia, like their adult counterparts, are susceptible to HIV-1 infection in vitro and can support the production of virus.
Full text
PDF

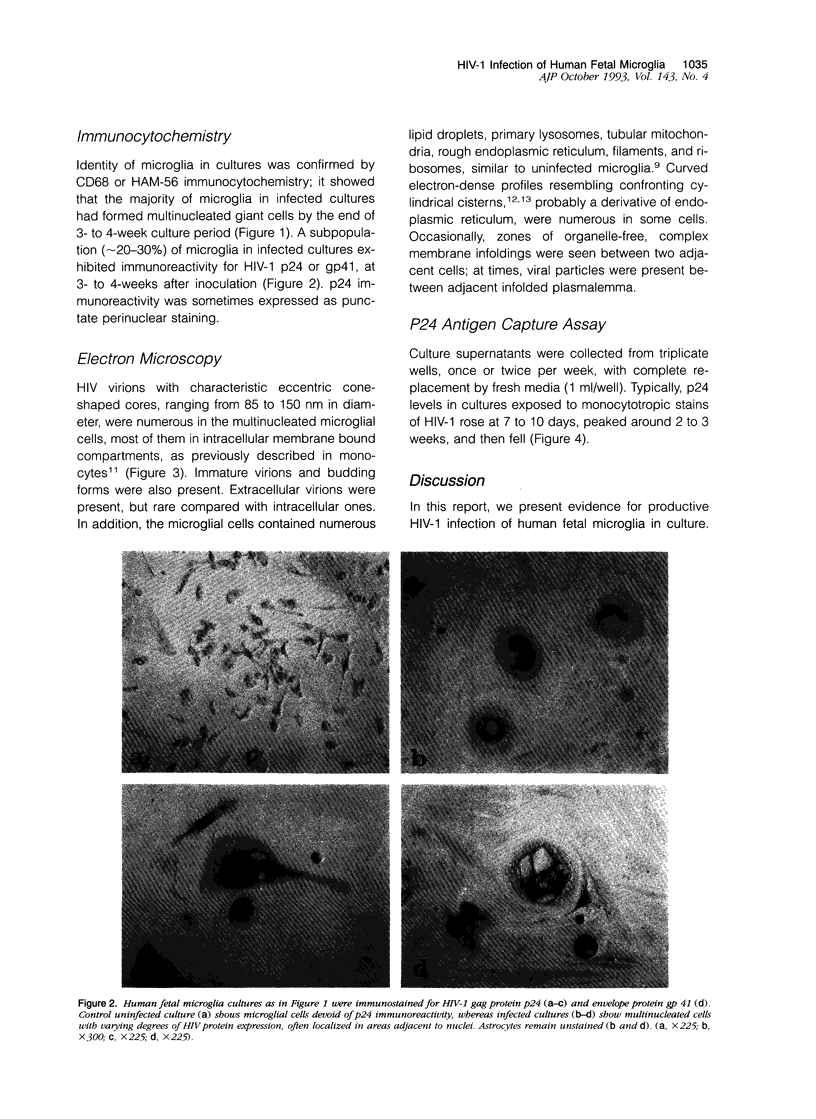
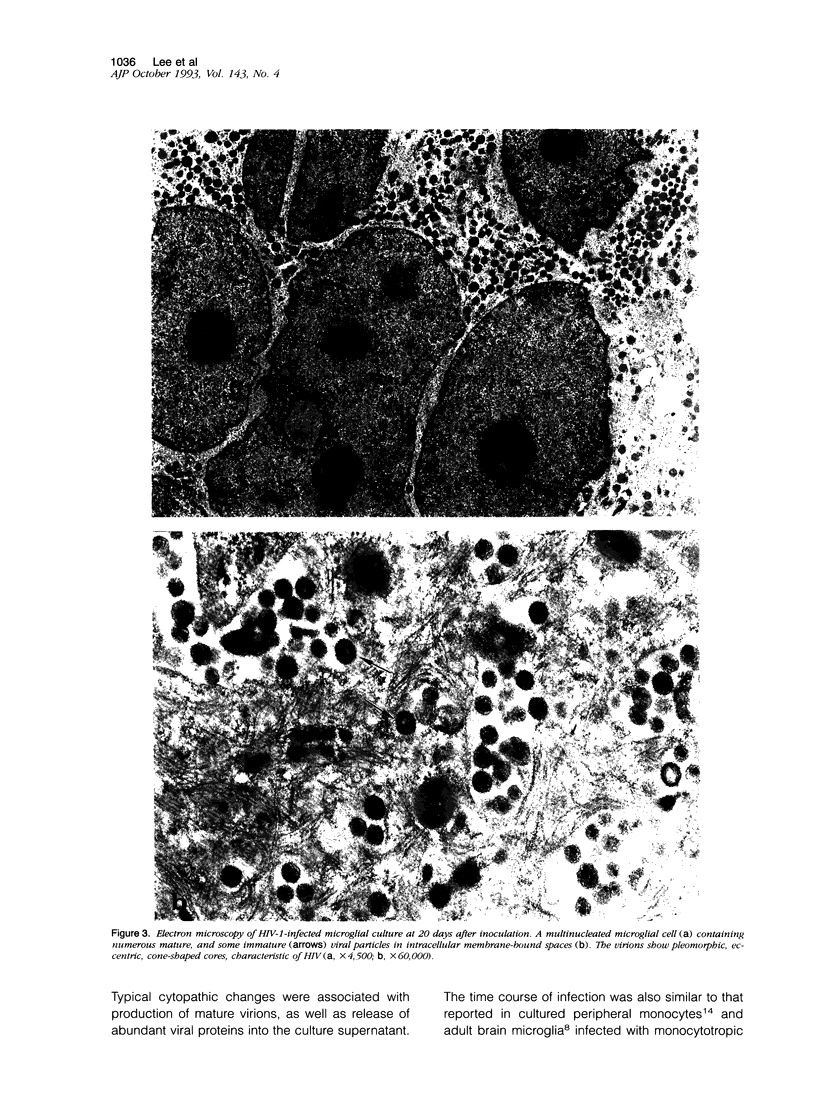
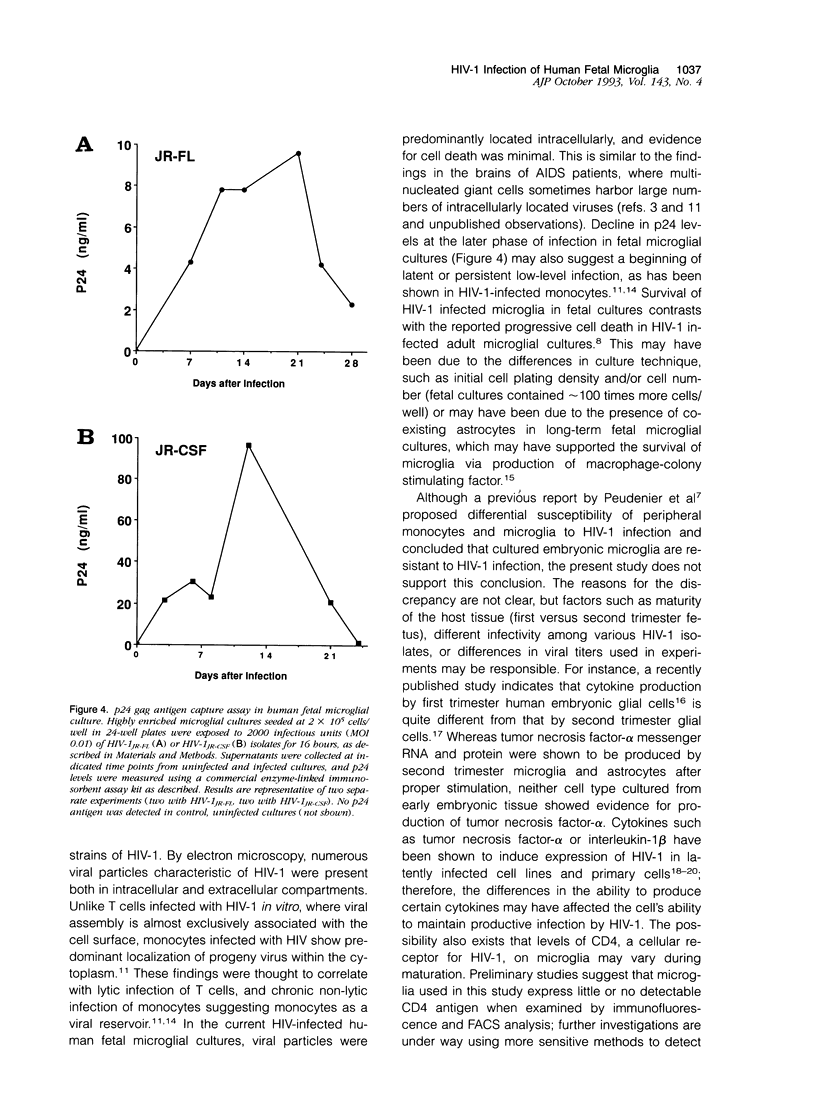


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Folks T. M., Clouse K. A., Justement J., Rabson A., Duh E., Kehrl J. H., Fauci A. S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Orenstein J. M., Martin M. A., Ferrua C., Mitra R., Phipps T., Wahl L. A., Lane H. C., Fauci A. S., Burke D. S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988 Apr 1;167(4):1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genis P., Jett M., Bernton E. W., Boyle T., Gelbard H. A., Dzenko K., Keane R. W., Resnick L., Mizrachi Y., Volsky D. J. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992 Dec 1;176(6):1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Vaca K., Noonan C. A. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990 Dec 14;250(4987):1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Kure K., Llena J. F., Lyman W. D., Soeiro R., Weidenheim K. M., Hirano A., Dickson D. W. Human immunodeficiency virus-1 infection of the nervous system: an autopsy study of 268 adult, pediatric, and fetal brains. Hum Pathol. 1991 Jul;22(7):700–710. doi: 10.1016/0046-8177(91)90293-x. [DOI] [PubMed] [Google Scholar]
- Lee S. C., Liu W., Brosnan C. F., Dickson D. W. Characterization of primary human fetal dissociated central nervous system cultures with an emphasis on microglia. Lab Invest. 1992 Oct;67(4):465–476. [PubMed] [Google Scholar]
- Lee S. C., Liu W., Dickson D. W., Brosnan C. F., Berman J. W. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol. 1993 Apr 1;150(7):2659–2667. [PubMed] [Google Scholar]
- Lee S. C., Liu W., Roth P., Dickson D. W., Berman J. W., Brosnan C. F. Macrophage colony-stimulating factor in human fetal astrocytes and microglia. Differential regulation by cytokines and lipopolysaccharide, and modulation of class II MHC on microglia. J Immunol. 1993 Jan 15;150(2):594–604. [PubMed] [Google Scholar]
- Lee S., Harris C., Hirschfeld A., Dickson D. W. Cytomembranous inclusions in the brain of a patient with the acquired immunodeficiency syndrome. Acta Neuropathol. 1988;76(1):101–106. doi: 10.1007/BF00687686. [DOI] [PubMed] [Google Scholar]
- Merrill J. E., Koyanagi Y., Chen I. S. Interleukin-1 and tumor necrosis factor alpha can be induced from mononuclear phagocytes by human immunodeficiency virus type 1 binding to the CD4 receptor. J Virol. 1989 Oct;63(10):4404–4408. doi: 10.1128/jvi.63.10.4404-4408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill J. E., Koyanagi Y., Zack J., Thomas L., Martin F., Chen I. S. Induction of interleukin-1 and tumor necrosis factor alpha in brain cultures by human immunodeficiency virus type 1. J Virol. 1992 Apr;66(4):2217–2225. doi: 10.1128/jvi.66.4.2217-2225.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navia B. A., Cho E. S., Petito C. K., Price R. W. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986 Jun;19(6):525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Navia B. A., Jordan B. D., Price R. W. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986 Jun;19(6):517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- Orenstein J. M., Meltzer M. S., Phipps T., Gendelman H. E. Cytoplasmic assembly and accumulation of human immunodeficiency virus types 1 and 2 in recombinant human colony-stimulating factor-1-treated human monocytes: an ultrastructural study. J Virol. 1988 Aug;62(8):2578–2586. doi: 10.1128/jvi.62.8.2578-2586.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein J. M., Schulof R. S., Simon G. L. Ultrastructural markers in acquired immune deficiency syndrome. Arch Pathol Lab Med. 1984 Nov;108(11):857–859. [PubMed] [Google Scholar]
- Peudenier S., Hery C., Montagnier L., Tardieu M. Human microglial cells: characterization in cerebral tissue and in primary culture, and study of their susceptibility to HIV-1 infection. Ann Neurol. 1991 Feb;29(2):152–161. doi: 10.1002/ana.410290207. [DOI] [PubMed] [Google Scholar]
- Poli G., Kinter A., Justement J. S., Kehrl J. H., Bressler P., Stanley S., Fauci A. S. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci U S A. 1990 Jan;87(2):782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L., Herndier B. G., Tang N. M., McGrath M. S. Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains. J Clin Invest. 1991 Feb;87(2):503–512. doi: 10.1172/JCI115024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharer L. R., Epstein L. G., Cho E. S., Joshi V. V., Meyenhofer M. F., Rankin L. F., Petito C. K. Pathologic features of AIDS encephalopathy in children: evidence for LAV/HTLV-III infection of brain. Hum Pathol. 1986 Mar;17(3):271–284. doi: 10.1016/s0046-8177(83)80220-2. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Rubinstein A., Rashbaum W. K., Lyman W. D. Maternofetal transmission of AIDS: frequency of human immunodeficiency virus type 1 nucleic acid sequences in human fetal DNA. J Infect Dis. 1992 Oct;166(4):699–703. doi: 10.1093/infdis/166.4.699. [DOI] [PubMed] [Google Scholar]
- Sébire G., Emilie D., Wallon C., Héry C., Devergne O., Delfraissy J. F., Galanaud P., Tardieu M. In vitro production of IL-6, IL-1 beta, and tumor necrosis factor-alpha by human embryonic microglial and neural cells. J Immunol. 1993 Feb 15;150(4):1517–1523. [PubMed] [Google Scholar]
- Tornatore C., Nath A., Amemiya K., Major E. O. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol. 1991 Nov;65(11):6094–6100. doi: 10.1128/jvi.65.11.6094-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazeux R., Lacroix-Ciaudo C., Blanche S., Cumont M. C., Henin D., Gray F., Boccon-Gibod L., Tardieu M. Low levels of human immunodeficiency virus replication in the brain tissue of children with severe acquired immunodeficiency syndrome encephalopathy. Am J Pathol. 1992 Jan;140(1):137–144. [PMC free article] [PubMed] [Google Scholar]
- Watkins B. A., Dorn H. H., Kelly W. B., Armstrong R. C., Potts B. J., Michaels F., Kufta C. V., Dubois-Dalcq M. Specific tropism of HIV-1 for microglial cells in primary human brain cultures. Science. 1990 Aug 3;249(4968):549–553. doi: 10.1126/science.2200125. [DOI] [PubMed] [Google Scholar]