Abstract
For many years, observation chambers, implanted in various animal species and in man, have been used for intravital microscopy of the microcirculation in granulation tissue, preformed tissue, or of the microvascularization of tissue implants. We describe herein a skinfold chamber model for the intravital microscopic investigation of striated skin muscle in immunoincompetent, nude mice over a minimum period of 2 weeks. Using fluorescent markers for contrast enhancement of plasma (fluorescein isothiocyanate-dextran) and leukocytes (acridine orange), the presented model allows the quantitative analysis of 1) the microhemodynamic parameters microvessel diameter and red blood cell velocity in arterioles (16 to 50 mu diameter), capillaries (4 to 9 mu diameter), and post-capillary venules (19 to 60 mu diameter), 2) leukocyte/endothelium interaction in these vessel segments, 3) functional capillary density and intercapillary distance, and 4) endothelial cell integrity. These parameters can be assessed in the microcirculation of the striated muscle tissue under normal or pathological conditions, as well as in the microcirculation of transplanted xenogeneic (human) neoplastic and non-neoplastic tissue grafts.
Full text
PDF

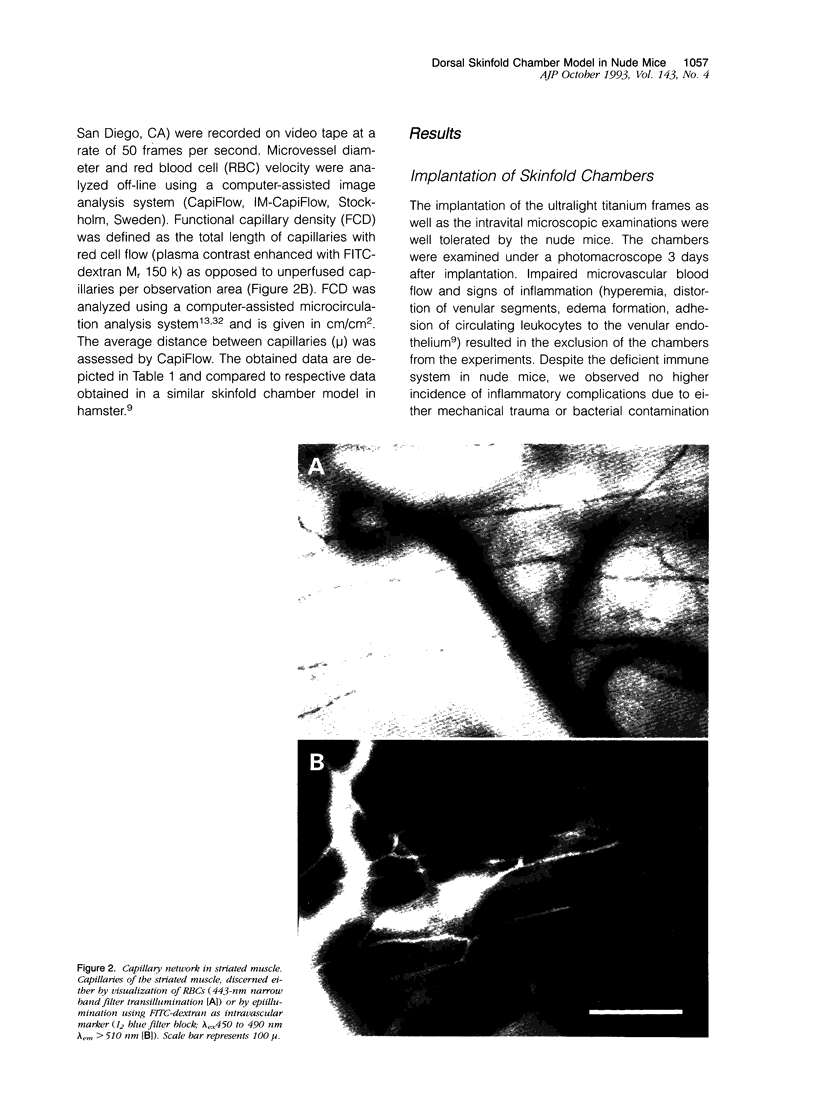
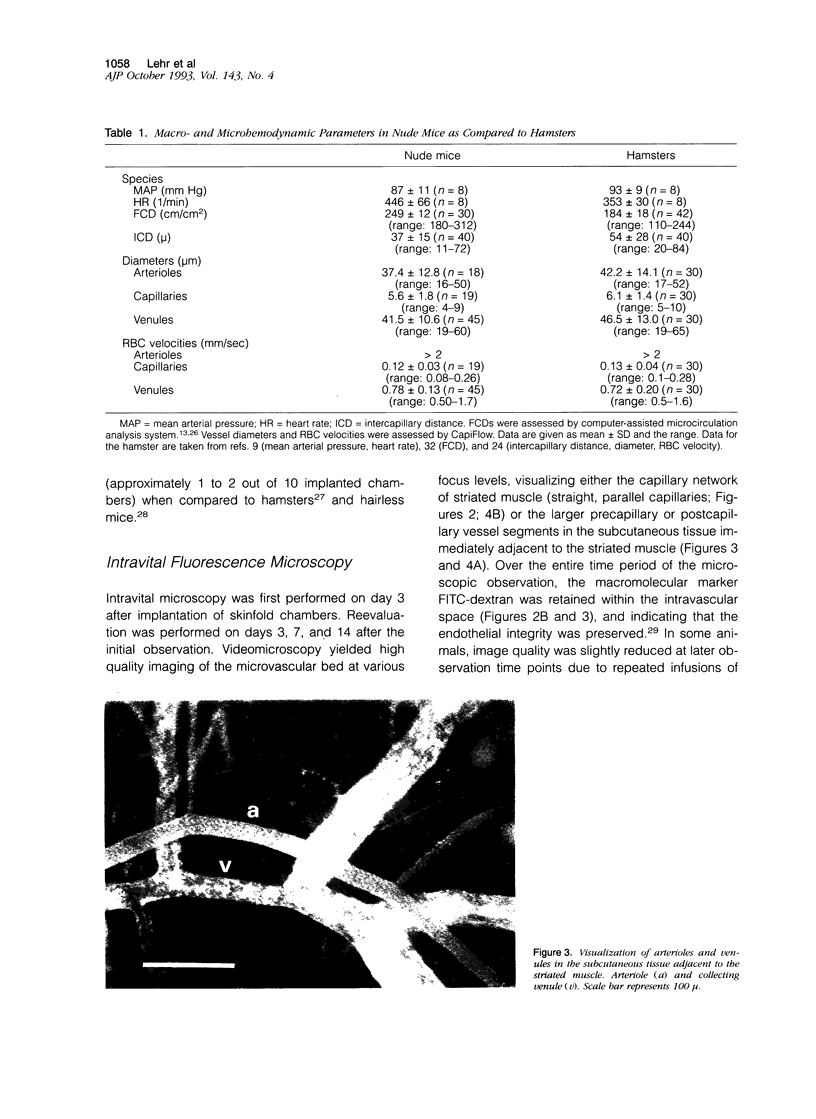
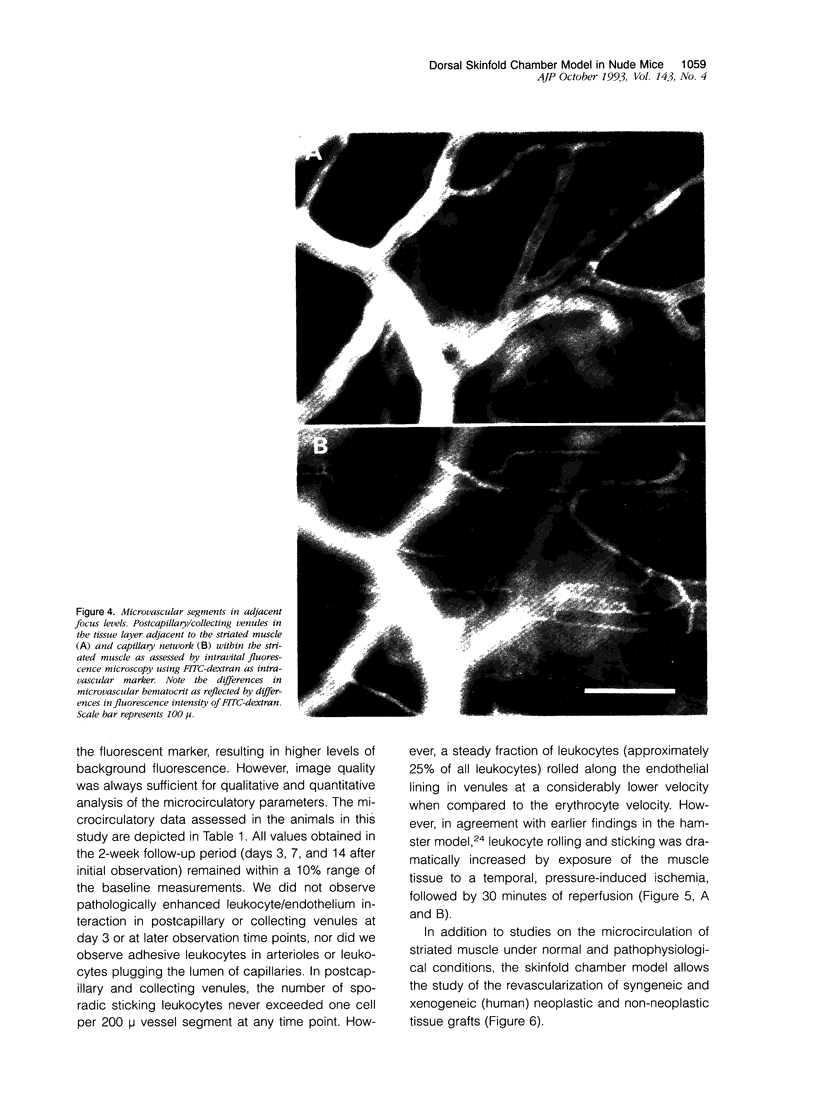
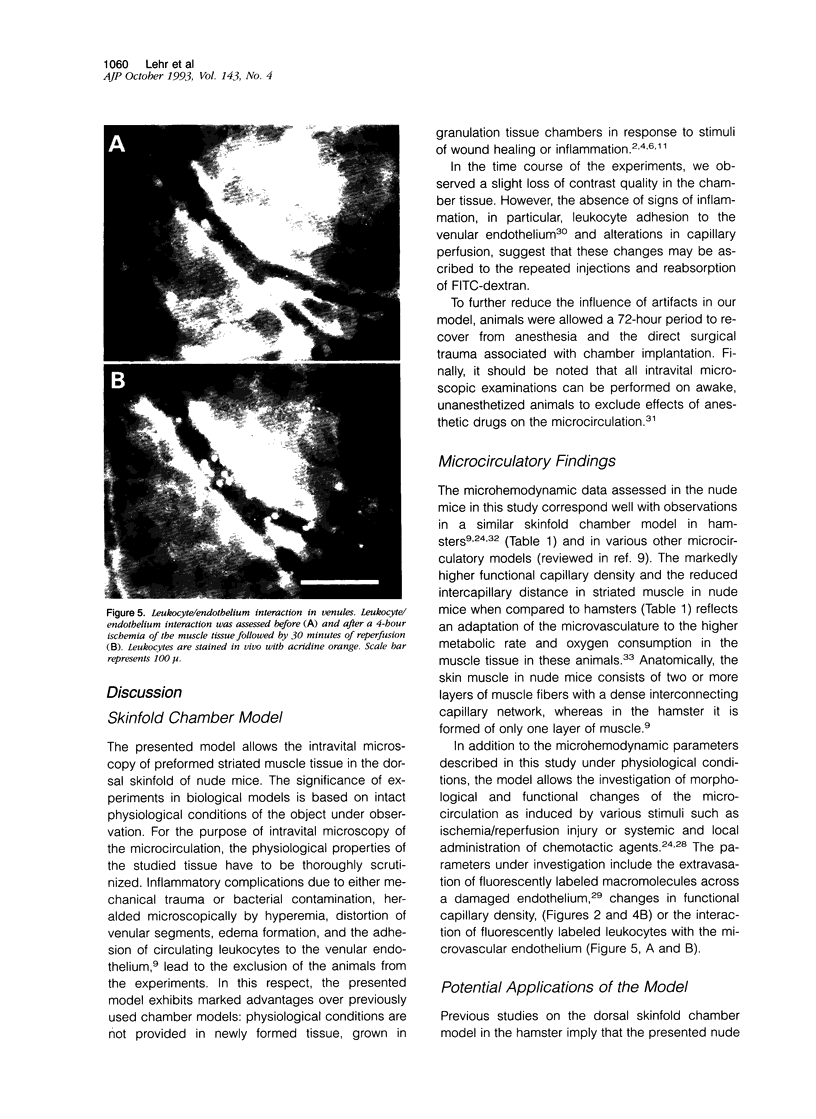


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASPEGREN K., BREINE U. MICROCIRCULATORY STUDIES IN MAN BY HIGH RESOLUTION VITAL MICROSCOPY. Angiology. 1964 Aug;15:329–332. doi: 10.1177/000331976401500801. [DOI] [PubMed] [Google Scholar]
- Arfors K. E., Jonsson J. A., McKenzie F. N. A titanium rabbit ear chamber: assembly, insertion and results. Microvasc Res. 1970 Oct;2(4):516–518. doi: 10.1016/0026-2862(70)90045-2. [DOI] [PubMed] [Google Scholar]
- Cardon S. Z., Oestermeyer C. F., Bloch E. H. Effect of oxygen on cyclic red blood cell flow in unanesthetized mammalian striated muscle as determined by microscopy. Microvasc Res. 1970 Jan;2(1):67–76. doi: 10.1016/0026-2862(70)90052-x. [DOI] [PubMed] [Google Scholar]
- Dillman R. O. Monoclonal antibodies for treating cancer. Ann Intern Med. 1989 Oct 1;111(7):592–603. doi: 10.7326/0003-4819-111-7-592. [DOI] [PubMed] [Google Scholar]
- Dumenco L. L., Arce C., Norton K., Yun J., Wagner T., Gerson S. L. Enhanced repair of O6-methylguanine DNA adducts in the liver of transgenic mice expressing the ada gene. Cancer Res. 1991 Jul 1;51(13):3391–3398. [PubMed] [Google Scholar]
- Endrich B., Asaishi K., Götz A., Messmer K. Technical report--a new chamber technique for microvascular studies in unanesthetized hamsters. Res Exp Med (Berl) 1980;177(2):125–134. doi: 10.1007/BF01851841. [DOI] [PubMed] [Google Scholar]
- Endrich B., Hammersen F., Messmer K. Hyperthermia-induced changes in tumor microcirculation. Recent Results Cancer Res. 1988;107:44–59. doi: 10.1007/978-3-642-83260-4_7. [DOI] [PubMed] [Google Scholar]
- Falkvoll K. H., Rofstad E. K., Brustad T., Marton P. A transparent chamber for the dorsal skin fold of athymic mice. Exp Cell Biol. 1984;52(4):260–268. doi: 10.1159/000163269. [DOI] [PubMed] [Google Scholar]
- Funk W., Endrich B., Messmer K. A novel method for follow-up studies of the microcirculation in non-malignant tissue implants. Res Exp Med (Berl) 1986;186(4):259–270. doi: 10.1007/BF01852303. [DOI] [PubMed] [Google Scholar]
- Ghose T., Ferrone S., Imai K., Norvell S. T., Jr, Luner S. J., Martin R. H., Blair A. H. Imaging of human melanoma xenografts in nude mice with a radiolabeled monoclonal antibody. J Natl Cancer Inst. 1982 Oct;69(4):823–826. [PubMed] [Google Scholar]
- Giovanella B. C., Stehlin J. S. Heterotransplantation of human malignant tumors in "nude" thymusless mice. I. Breeding and maintenance of "nude" mice. J Natl Cancer Inst. 1973 Aug;51(2):615–619. [PubMed] [Google Scholar]
- Hobbs J. B., Chusilp S., Hua A., Kincaid-Smith P., McIver M. A. The pathogenesis of hypertensive vascular changes in the rat: microscopic and ultrastructural correlation in vivo. Clin Sci Mol Med Suppl. 1976 Dec;3:73s–75s. doi: 10.1042/cs051073s. [DOI] [PubMed] [Google Scholar]
- Lehr H. A., Guhlmann A., Nolte D., Keppler D., Messmer K. Leukotrienes as mediators in ischemia-reperfusion injury in a microcirculation model in the hamster. J Clin Invest. 1991 Jun;87(6):2036–2041. doi: 10.1172/JCI115233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehr H. A., Hübner C., Nolte D., Kohlschütter A., Messmer K. Dietary fish oil blocks the microcirculatory manifestations of ischemia-reperfusion injury in striated muscle in hamsters. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6726–6730. doi: 10.1073/pnas.88.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehr H. A., Kröber M., Hübner C., Vajkoczy P., Menger M. D., Nolte D., Kohlschütter A., Messmer K. Stimulation of leukocyte/endothelium interaction by oxidized low-density lipoprotein in hairless mice. Involvement of CD11b/CD18 adhesion receptor complex. Lab Invest. 1993 Apr;68(4):388–395. [PubMed] [Google Scholar]
- Longnecker D. E., Harris P. D. Microcirculatory actions of general anesthetics. Fed Proc. 1980 Apr;39(5):1580–1583. [PubMed] [Google Scholar]
- Menger M. D., Jäger S., Walter P., Hammersen F., Messmer K. A novel technique for studies on the microvasculature of transplanted islets of Langerhans in vivo. Int J Microcirc Clin Exp. 1990 Feb;9(1):103–117. [PubMed] [Google Scholar]
- Menger M. D., Pattenier J., Wolf B., Jäger S., Feifel G., Messmer K. Cryopreservation of islets of Langerhans does not affect angiogenesis and revascularization after free transplantation. Eur Surg Res. 1992;24(2):89–96. doi: 10.1159/000129193. [DOI] [PubMed] [Google Scholar]
- Menger M. D., Sack F. U., Barker J. H., Feifel G., Messmer K. Quantitative analysis of microcirculatory disorders after prolonged ischemia in skeletal muscle. Therapeutic effects of prophylactic isovolemic hemodilution. Res Exp Med (Berl) 1988;188(3):151–165. doi: 10.1007/BF01852316. [DOI] [PubMed] [Google Scholar]
- Oda T., Lehmann A., Endrich B. Capillary blood flow in the amelanotic melanoma of the hamster after isovolemic hemodilution. Biorheology. 1984;21(4):509–520. doi: 10.3233/bir-1984-21410. [DOI] [PubMed] [Google Scholar]
- Papenfuss H. D., Gross J. F., Intaglietta M., Treese F. A. A transparent access chamber for the rat dorsal skin fold. Microvasc Res. 1979 Nov;18(3):311–318. doi: 10.1016/0026-2862(79)90039-6. [DOI] [PubMed] [Google Scholar]
- Prewitt R. L., Johnson P. C. The effect of oxygen on arteriolar red cell velocity and capillary density in the rat cremaster muscle. Microvasc Res. 1976 Jul;12(1):59–70. doi: 10.1016/0026-2862(76)90007-8. [DOI] [PubMed] [Google Scholar]
- SCHMIDT-NIELSEN K., PENNYCUIK P. Capillary density in mammals in relation to body size and oxygen consumption. Am J Physiol. 1961 Apr;200:746–750. doi: 10.1152/ajplegacy.1961.200.4.746. [DOI] [PubMed] [Google Scholar]
- Sacchi M., Vitolo D., Sedlmayr P., Rabinowich H., Johnson J. T., Herberman R. B., Whiteside T. L. Induction of tumor regression in experimental model of human head and neck cancer by human A-LAK cells and IL-2. Int J Cancer. 1991 Mar 12;47(5):784–791. doi: 10.1002/ijc.2910470527. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeintl H., Sack F. U., Intaglietta M., Messmer K. Computer assisted leukocyte adhesion measurement in intravital microscopy. Int J Microcirc Clin Exp. 1989 Jul;8(3):293–302. [PubMed] [Google Scholar]