Abstract
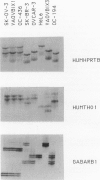
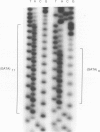
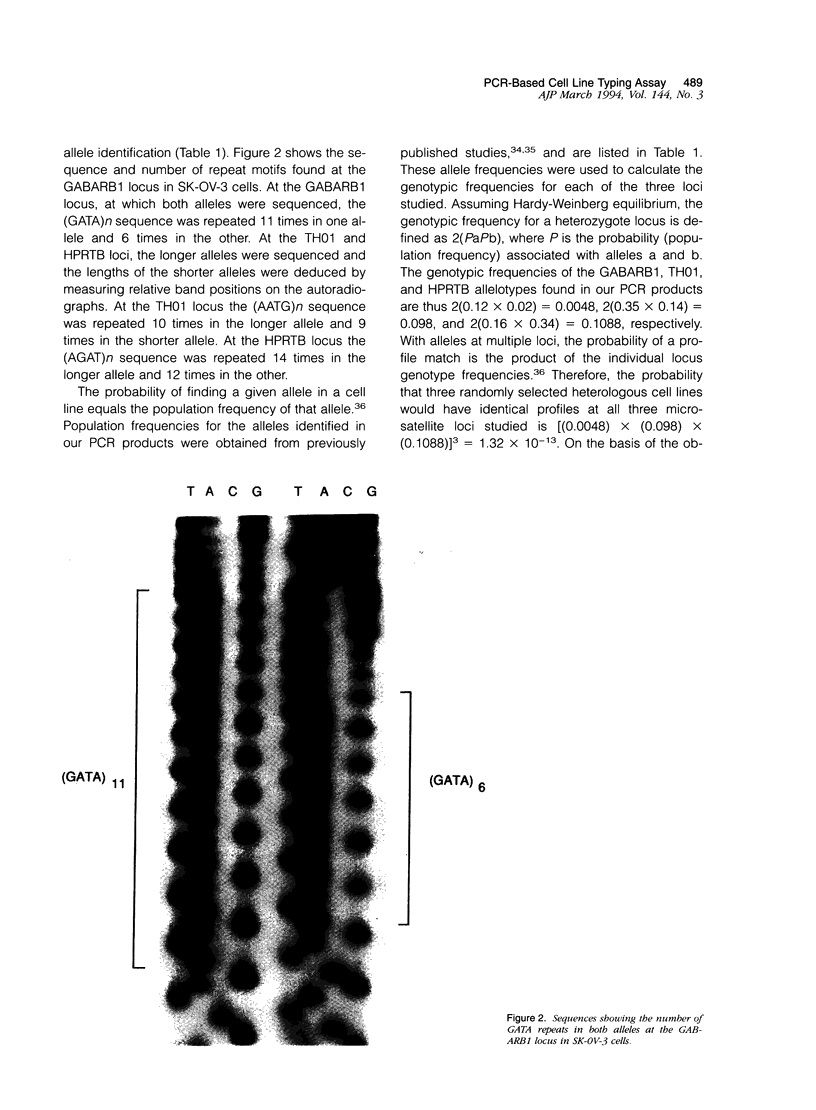
In this report we describe the application of a polymerase chain reaction (PCR)-based DNA typing assay to the analysis of tumor cell line identity. We have applied the technique to analyze four tumor cell lines purchased from the American Type Culture Collection (SK-OV-3, SK-BR-3, OVCAR, HeLa) and four lines isolated from the ascites fluids of ovarian cancer patients (YAOVBIX1, YAOVBIX3, OC194, and OC346). In this assay, three polymorphic tetranucleotide microsatellite loci (GABARB1, TH01, and HPRTB) were amplified from tumor cell line DNAs in radioactive PCR-reactions. The products were resolved in polyacrylamide gels and exposed to film to produce individual-specific patterns for five of the cell lines (HeLa, SK-BR-3, OVCAR, YAOVBIX3, and OC194). However, three of the cell lines, SK-OV-3, YAOVBIX1, and OC436 had identical "fingerprints" at all three loci. The probability that the observed profile match could occur between three randomly selected heterologous cell lines was calculated to be 1.32 x 10(-13). On the basis of this analysis, we have identified two independent cross-contamination events involving the SK-OV-3 ovarian adenocarcinoma cell line. The PCR-based analysis of tetranucleotide microsatellite loci is technically straightforward and produces discrete allelic bands associated with known population frequencies, allowing for the unequivocal interpretation of typing patterns.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Anglard P., Trahan E., Liu S., Latif F., Merino M. J., Lerman M. I., Zbar B., Linehan W. M. Molecular and cellular characterization of human renal cell carcinoma cell lines. Cancer Res. 1992 Jan 15;52(2):348–356. [PubMed] [Google Scholar]
- Band V., Zajchowski D., Swisshelm K., Trask D., Kulesa V., Cohen C., Connolly J., Sager R. Tumor progression in four mammary epithelial cell lines derived from the same patient. Cancer Res. 1990 Nov 15;50(22):7351–7357. [PubMed] [Google Scholar]
- Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988 Mar;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buick R. N., Pullano R., Trent J. M. Comparative properties of five human ovarian adenocarcinoma cell lines. Cancer Res. 1985 Aug;45(8):3668–3676. [PubMed] [Google Scholar]
- Dean M., Lucas-Derse S., Bolos A., O'Brien S. J., Kirkness E. F., Fraser C. M., Goldman D. Genetic mapping of the beta 1 GABA receptor gene to human chromosome 4, using a tetranucleotide repeat polymorphism. Am J Hum Genet. 1991 Sep;49(3):621–626. [PMC free article] [PubMed] [Google Scholar]
- Edwards A., Civitello A., Hammond H. A., Caskey C. T. DNA typing and genetic mapping with trimeric and tetrameric tandem repeats. Am J Hum Genet. 1991 Oct;49(4):746–756. [PMC free article] [PubMed] [Google Scholar]
- Edwards A., Hammond H. A., Jin L., Caskey C. T., Chakraborty R. Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups. Genomics. 1992 Feb;12(2):241–253. doi: 10.1016/0888-7543(92)90371-x. [DOI] [PubMed] [Google Scholar]
- Fey M. F., Tobler A. Assessment of DNA 'fingerprinting' as a method for validating the identity of cancer cell lines maintained in long-term culture. Nucleic Acids Res. 1991 Jun 25;19(12):3464–3464. doi: 10.1093/nar/19.12.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogh J., Fogh J. M., Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977 Jul;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- Gilbert D. A., Reid Y. A., Gail M. H., Pee D., White C., Hay R. J., O'Brien S. J. Application of DNA fingerprints for cell-line individualization. Am J Hum Genet. 1990 Sep;47(3):499–514. [PMC free article] [PubMed] [Google Scholar]
- Han H. J., Yanagisawa A., Kato Y., Park J. G., Nakamura Y. Genetic instability in pancreatic cancer and poorly differentiated type of gastric cancer. Cancer Res. 1993 Nov 1;53(21):5087–5089. [PubMed] [Google Scholar]
- Häne B., Tümmler M., Jäger K., Schleithoff L., Janssen J. W., Drexler H. G. Differences in DNA fingerprints of continuous leukemia-lymphoma cell lines from different sources. Leukemia. 1992 Nov;6(11):1129–1133. [PubMed] [Google Scholar]
- Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993 Jun 10;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Brookfield J. F., Semeonoff R. Positive identification of an immigration test-case using human DNA fingerprints. 1985 Oct 31-Nov 6Nature. 317(6040):818–819. doi: 10.1038/317818a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Individual-specific 'fingerprints' of human DNA. Nature. 1985 Jul 4;316(6023):76–79. doi: 10.1038/316076a0. [DOI] [PubMed] [Google Scholar]
- Kacinski B. M., Carter D., Mittal K., Yee L. D., Scata K. A., Donofrio L., Chambers S. K., Wang K. I., Yang-Feng T., Rohrschneider L. R. Ovarian adenocarcinomas express fms-complementary transcripts and fms antigen, often with coexpression of CSF-1. Am J Pathol. 1990 Jul;137(1):135–147. [PMC free article] [PubMed] [Google Scholar]
- King B. L., Carter D., Foellmer H. G., Kacinski B. M. Neu proto-oncogene amplification and expression in ovarian adenocarcinoma cell lines. Am J Pathol. 1992 Jan;140(1):23–31. [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein A., Berenson J., Gera J. F., Waldburger K., Martinez-Maza O., Berek J. S. Resistance of human ovarian cancer cells to tumor necrosis factor and lymphokine-activated killer cells: correlation with expression of HER2/neu oncogenes. Cancer Res. 1990 Nov 15;50(22):7364–7370. [PubMed] [Google Scholar]
- Morimoto H., Safrit J. T., Bonavida B. Synergistic effect of tumor necrosis factor-alpha- and diphtheria toxin-mediated cytotoxicity in sensitive and resistant human ovarian tumor cell lines. J Immunol. 1991 Oct 15;147(8):2609–2616. [PubMed] [Google Scholar]
- Murphy C. S., Pink J. J., Jordan V. C. Characterization of a receptor-negative, hormone-nonresponsive clone derived from a T47D human breast cancer cell line kept under estrogen-free conditions. Cancer Res. 1990 Nov 15;50(22):7285–7292. [PubMed] [Google Scholar]
- Nelson-Rees W. A., Daniels D. W., Flandermeyer R. R. Cross-contamination of cells in culture. Science. 1981 Apr 24;212(4493):446–452. doi: 10.1126/science.6451928. [DOI] [PubMed] [Google Scholar]
- Nelson-Rees W. A., Flandermeyer R. R., Hawthorne P. K. Banded marker chromosomes as indicators of intraspecies cellular contamination. Science. 1974 Jun 7;184(4141):1093–1096. doi: 10.1126/science.184.4141.1093. [DOI] [PubMed] [Google Scholar]
- Nelson-Rees W. A., Flandermeyer R. R. HeLa cultures defined. Science. 1976 Jan 9;191(4222):96–98. doi: 10.1126/science.1246601. [DOI] [PubMed] [Google Scholar]
- Nelson-Rees W. A., Flandermeyer R. R. Inter- and intraspecies contamination of human breast tumor cell lines HBC and BrCa5 and other cell cultures. Science. 1977 Mar 25;195(4284):1343–1344. doi: 10.1126/science.557237. [DOI] [PubMed] [Google Scholar]
- Nio Y., Zighelboim J., Berek J. S., Bonavida B. Sensitivity of fresh and cultured ovarian tumor cells to tumor necrosis factor, interferon-alpha 2, and OK-432. Cancer Immunol Immunother. 1988;27(3):246–254. doi: 10.1007/BF00205447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine T. M., Soule H. D., Pauley R. J., Dawson P. J. Characterization of epithelial phenotypes in mortal and immortal human breast cells. Int J Cancer. 1992 Feb 1;50(3):463–473. doi: 10.1002/ijc.2910500323. [DOI] [PubMed] [Google Scholar]
- Parsons R., Li G. M., Longley M. J., Fang W. H., Papadopoulos N., Jen J., de la Chapelle A., Kinzler K. W., Vogelstein B., Modrich P. Hypermutability and mismatch repair deficiency in RER+ tumor cells. Cell. 1993 Dec 17;75(6):1227–1236. doi: 10.1016/0092-8674(93)90331-j. [DOI] [PubMed] [Google Scholar]
- Risinger J. I., Berchuck A., Kohler M. F., Watson P., Lynch H. T., Boyd J. Genetic instability of microsatellites in endometrial carcinoma. Cancer Res. 1993 Nov 1;53(21):5100–5103. [PubMed] [Google Scholar]
- Stacey G. N., Bolton B. J., Doyle A. DNA fingerprinting transforms the art of cell authentication. Nature. 1992 May 21;357(6375):261–262. doi: 10.1038/357261a0. [DOI] [PubMed] [Google Scholar]
- Stromberg K., Collins T. J., 4th, Gordon A. W., Jackson C. L., Johnson G. R. Transforming growth factor-alpha acts as an autocrine growth factor in ovarian carcinoma cell lines. Cancer Res. 1992 Jan 15;52(2):341–347. [PubMed] [Google Scholar]
- Thacker J., Webb M. B., Debenham P. G. Fingerprinting cell lines: use of human hypervariable DNA probes to characterize mammalian cell cultures. Somat Cell Mol Genet. 1988 Nov;14(6):519–525. doi: 10.1007/BF01535307. [DOI] [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Watson J. M., Sensintaffar J. L., Berek J. S., Martínez-Maza O. Constitutive production of interleukin 6 by ovarian cancer cell lines and by primary ovarian tumor cultures. Cancer Res. 1990 Nov 1;50(21):6959–6965. [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- van Helden P. D., Wiid I. J., Albrecht C. F., Theron E., Thornley A. L., Hoal-van Helden E. G. Cross-contamination of human esophageal squamous carcinoma cell lines detected by DNA fingerprint analysis. Cancer Res. 1988 Oct 15;48(20):5660–5662. [PubMed] [Google Scholar]