Abstract
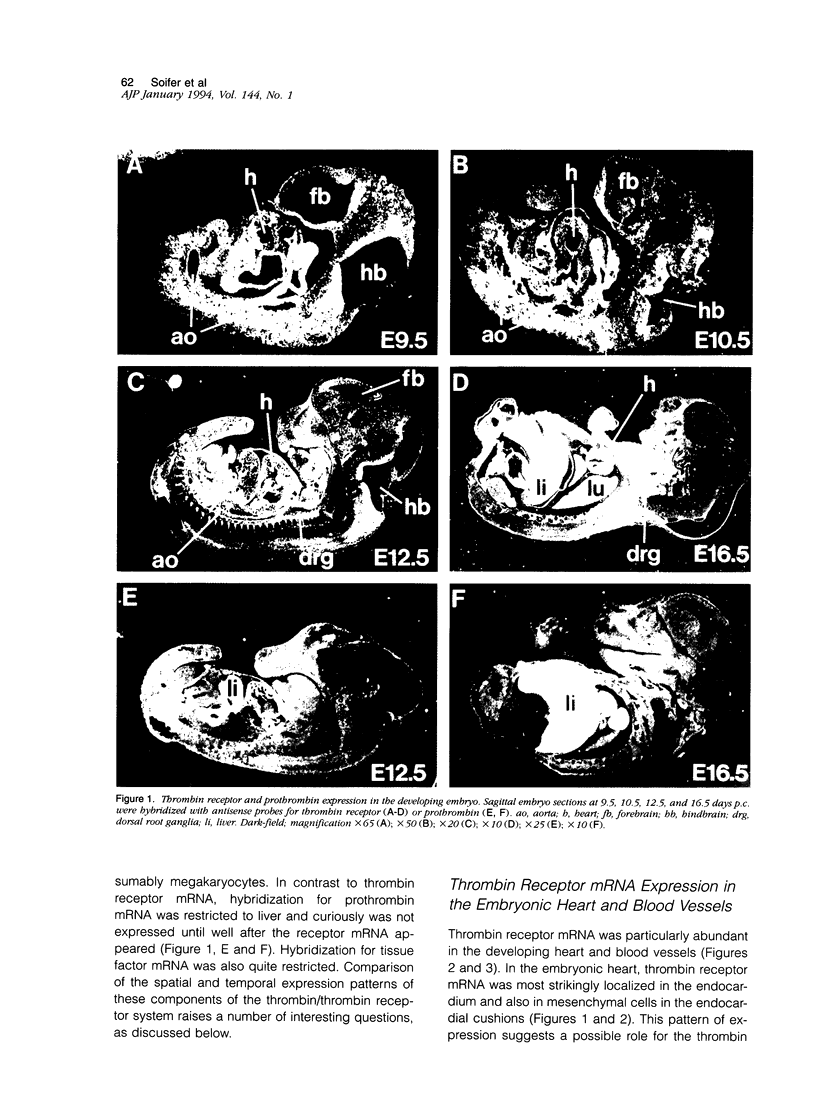
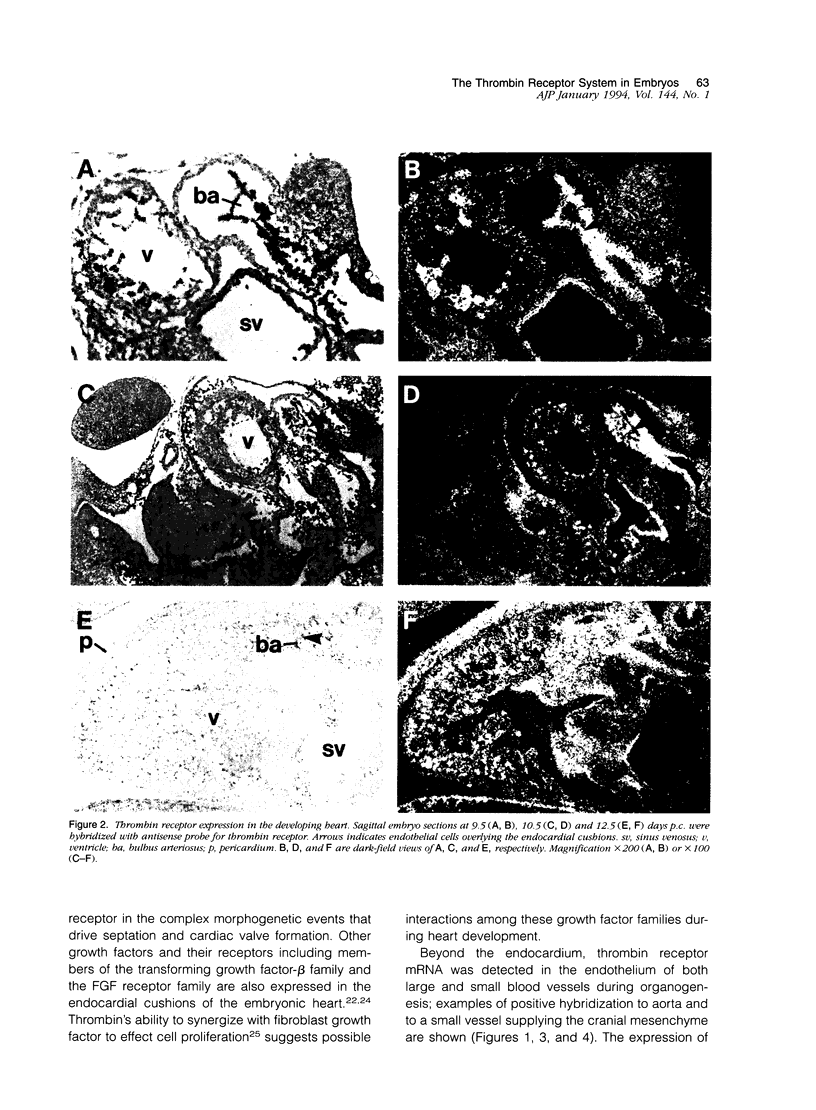
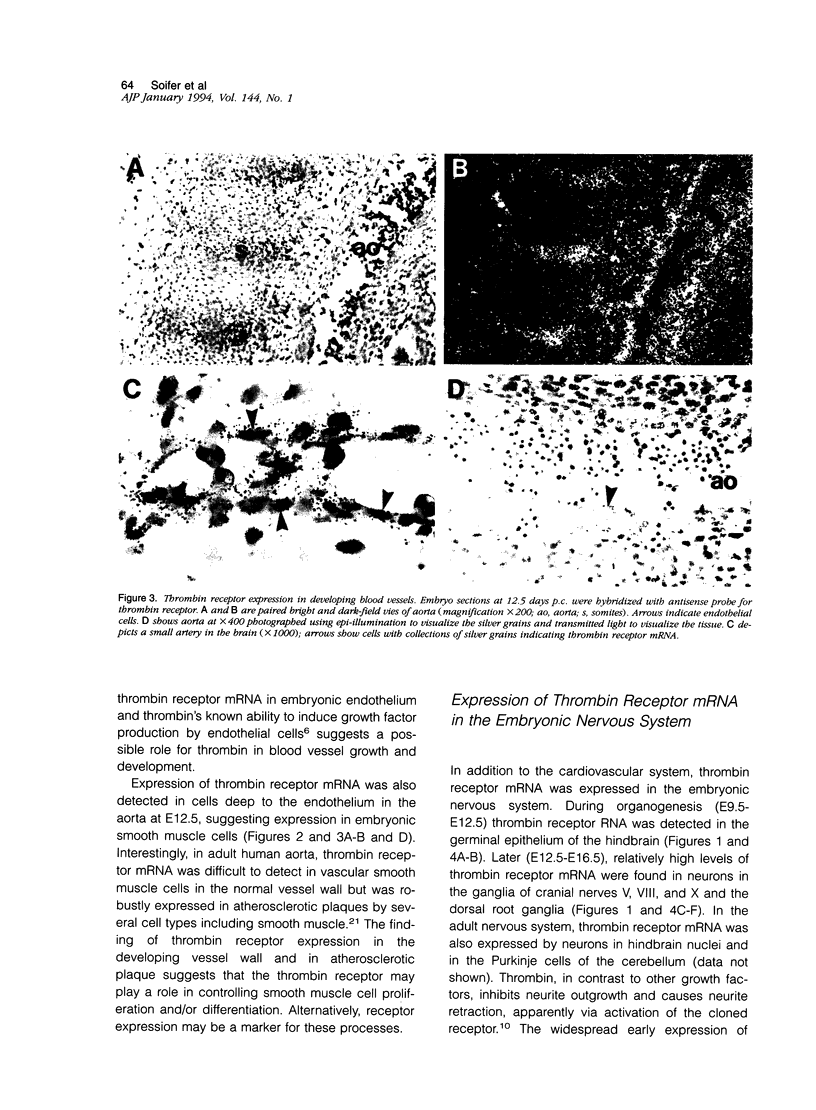
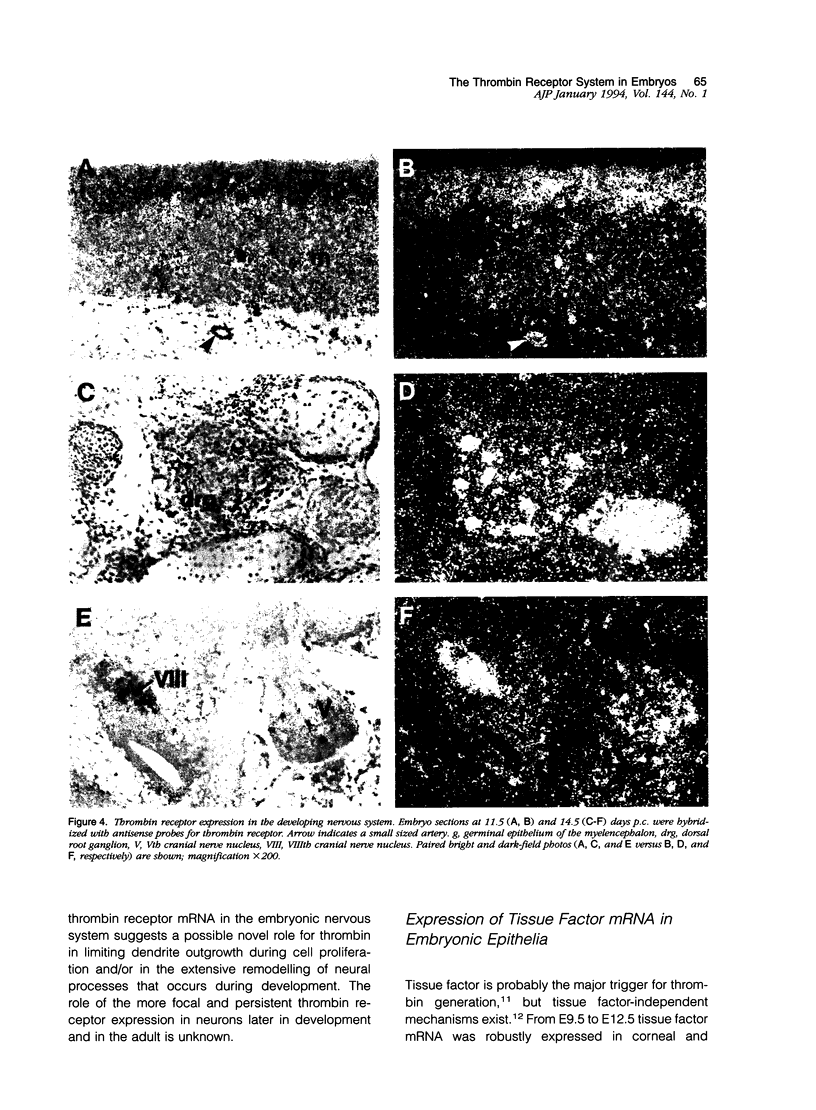
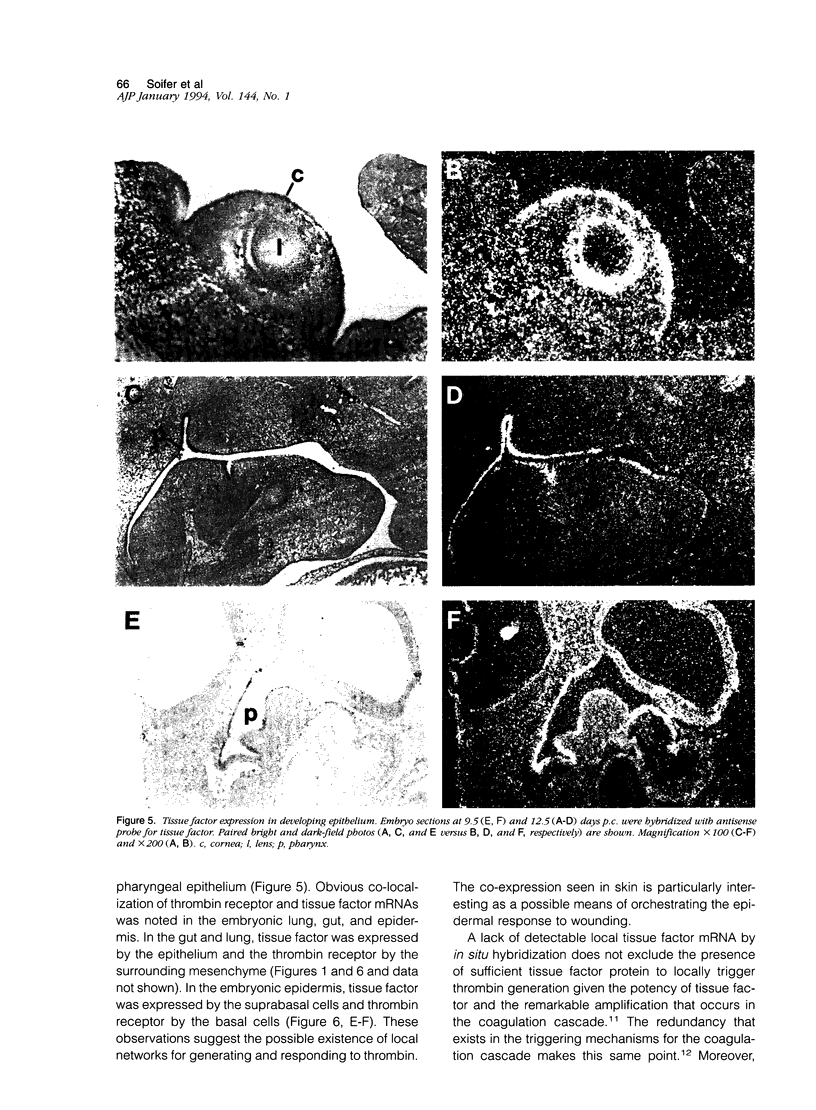
The protease thrombin is a potent agonist for platelet aggregation, mesenchymal cell proliferation, and endothelial production of growth factors and adhesion molecules. Thrombin also modulates neurite outgrowth in neuronal cultures. These apparently disparate responses to thrombin appear to be largely mediated by the recently cloned thrombin receptor. In the adult, thrombin is generated from its zymogen prothrombin at sites of vascular injury when circulating coagulation factors meet extravascular tissue factor. In this context thrombin's varied actions may mediate responses to wounding. Whether thrombin's actions on cells may also play a role in development is unknown. We examined the expression of thrombin receptor, prothrombin, and tissue factor by in situ hybridization in mouse development. Thrombin receptor mRNA was expressed widely in mesenchymal cell populations during early organogenesis (E9.5) and was particularly abundant in developing heart and blood vessels. Robust receptor expression was also noted in the germinal epithelium of the hindbrain. Thrombin receptor expression became more restricted with time and by the fetal growth stage (E16.5) was most readily detected in certain neurons, endocardial and endothelial cells, and within lung and liver. In contrast to the thrombin receptor, prothrombin mRNA was limited to the embryonic liver and was not detected until E12.5, well after the onset of receptor expression. mRNA for tissue factor, one important trigger for thrombin generation in the adult, was detected in embryonic epithelia from E9.5-12.5. In several instances, tissue factor-expressing epithelia were surrounded by thrombin receptor-expressing mesenchyme. These data suggest a possible role for the thrombin receptor in development. The finding of robust thrombin receptor expression before prothrombin mRNA was detected raises the question of whether other proteases or peptide ligands can activate the thrombin receptor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit R., Kahn A., Wilner G. D., Fenton J. W., 2nd Monocyte chemotaxis: stimulation by specific exosite region in thrombin. Science. 1983 May 13;220(4598):728–731. doi: 10.1126/science.6836310. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):131–135. doi: 10.1073/pnas.72.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. O., Gibbs V. C., Milfay D. F., Garovoy M. R., Williams L. T. Thrombin stimulates c-sis gene expression in microvascular endothelial cells. J Biol Chem. 1986 Jul 25;261(21):9579–9582. [PubMed] [Google Scholar]
- Davey M. G., Lüscher E. F. Actions of thrombin and other coagulant and proteolytic enzymes on blood platelets. Nature. 1967 Dec 2;216(5118):857–858. doi: 10.1038/216857a0. [DOI] [PubMed] [Google Scholar]
- Degen S. J., Schaefer L. A., Jamison C. S., Grant S. G., Fitzgibbon J. J., Pai J. A., Chapman V. M., Elliott R. W. Characterization of the cDNA coding for mouse prothrombin and localization of the gene on mouse chromosome 2. DNA Cell Biol. 1990 Sep;9(7):487–498. doi: 10.1089/dna.1990.9.487. [DOI] [PubMed] [Google Scholar]
- Dickson M. C., Slager H. G., Duffie E., Mummery C. L., Akhurst R. J. RNA and protein localisations of TGF beta 2 in the early mouse embryo suggest an involvement in cardiac development. Development. 1993 Feb;117(2):625–639. doi: 10.1242/dev.117.2.625. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd Regulation of thrombin generation and functions. Semin Thromb Hemost. 1988 Jul;14(3):234–240. doi: 10.1055/s-2007-1002783. [DOI] [PubMed] [Google Scholar]
- Forestier F., Daffos F., Rainaut M., Solé Y., Amiral J. Vitamin K dependent proteins in fetal hemostasis at mid trimester of pregnancy. Thromb Haemost. 1985 Jun 24;53(3):401–403. [PubMed] [Google Scholar]
- Frohman M. A., Boyle M., Martin G. R. Isolation of the mouse Hox-2.9 gene; analysis of embryonic expression suggests that positional information along the anterior-posterior axis is specified by mesoderm. Development. 1990 Oct;110(2):589–607. doi: 10.1242/dev.110.2.589. [DOI] [PubMed] [Google Scholar]
- Gelehrter T. D., Sznycer-Laszuk R. Thrombin induction of plasminogen activator-inhibitor in cultured human endothelial cells. J Clin Invest. 1986 Jan;77(1):165–169. doi: 10.1172/JCI112271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung D. T., Vu T. H., Nelken N. A., Coughlin S. R. Thrombin-induced events in non-platelet cells are mediated by the unique proteolytic mechanism established for the cloned platelet thrombin receptor. J Cell Biol. 1992 Feb;116(3):827–832. doi: 10.1083/jcb.116.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung D. T., Vu T. K., Wheaton V. I., Ishii K., Coughlin S. R. Cloned platelet thrombin receptor is necessary for thrombin-induced platelet activation. J Clin Invest. 1992 Apr;89(4):1350–1353. doi: 10.1172/JCI115721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison C. S., Degen S. J. Prenatal and postnatal expression of mRNA coding for rat prothrombin. Biochim Biophys Acta. 1991 Feb 16;1088(2):208–216. doi: 10.1016/0167-4781(91)90056-r. [DOI] [PubMed] [Google Scholar]
- Joseph S., MacDermot J. The N-terminal thrombin receptor fragment SFLLRN, but not catalytically inactive thrombin-derived agonists, activate U937 human monocytic cells: evidence for receptor hydrolysis in thrombin-dependent signalling. Biochem J. 1993 Mar 1;290(Pt 2):571–577. doi: 10.1042/bj2900571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara C. A., Sarembock I. J., Gimple L. W., Fenton J. W., 2nd, Coughlin S. R., Owens G. K. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J Clin Invest. 1993 Jan;91(1):94–98. doi: 10.1172/JCI116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelken N. A., Soifer S. J., O'Keefe J., Vu T. K., Charo I. F., Coughlin S. R. Thrombin receptor expression in normal and atherosclerotic human arteries. J Clin Invest. 1992 Oct;90(4):1614–1621. doi: 10.1172/JCI116031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerson Y. The tissue factor pathway of blood coagulation. Semin Hematol. 1992 Jul;29(3):170–176. [PubMed] [Google Scholar]
- Ngaiza J. R., Jaffe E. A. A 14 amino acid peptide derived from the amino terminus of the cleaved thrombin receptor elevates intracellular calcium and stimulates prostacyclin production in human endothelial cells. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1656–1661. doi: 10.1016/0006-291x(91)91765-5. [DOI] [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Mitogenic effects of fibroblast growth factors in cultured fibroblasts. Interaction with the G-protein-mediated signaling pathways. Ann N Y Acad Sci. 1991;638:139–148. doi: 10.1111/j.1749-6632.1991.tb49024.x. [DOI] [PubMed] [Google Scholar]
- Peters K. G., Werner S., Chen G., Williams L. T. Two FGF receptor genes are differentially expressed in epithelial and mesenchymal tissues during limb formation and organogenesis in the mouse. Development. 1992 Jan;114(1):233–243. doi: 10.1242/dev.114.1.233. [DOI] [PubMed] [Google Scholar]
- Prescott S. M., Zimmerman G. A., McIntyre T. M. Human endothelial cells in culture produce platelet-activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when stimulated with thrombin. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3534–3538. doi: 10.1073/pnas.81.11.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan G., Blatti S. P., Subramaniam M., Fass D. N., Maihle N. J., Getz M. J. Cloning of murine tissue factor and regulation of gene expression by transforming growth factor type beta 1. J Biol Chem. 1991 Jan 5;266(1):496–501. [PubMed] [Google Scholar]
- Suidan H. S., Stone S. R., Hemmings B. A., Monard D. Thrombin causes neurite retraction in neuronal cells through activation of cell surface receptors. Neuron. 1992 Feb;8(2):363–375. doi: 10.1016/0896-6273(92)90302-t. [DOI] [PubMed] [Google Scholar]
- Terwiel J., Veltkamp J. J., Bertina R. M., Muller H. P. Coagulation factors in the human fetus of about 20 weeks of gestational age. Br J Haematol. 1980 Aug;45(4):641–650. doi: 10.1111/j.1365-2141.1980.tb07187.x. [DOI] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]