Abstract
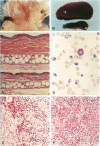
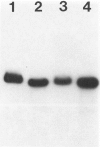
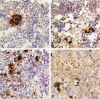
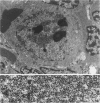
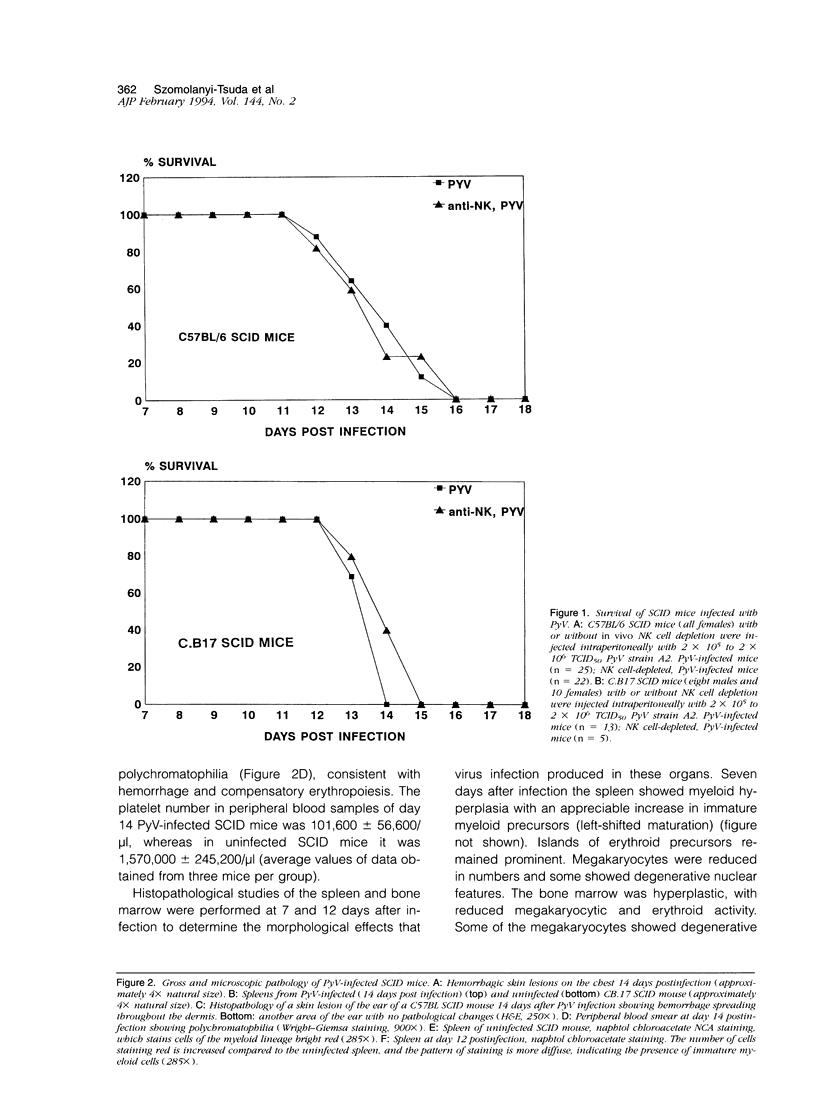
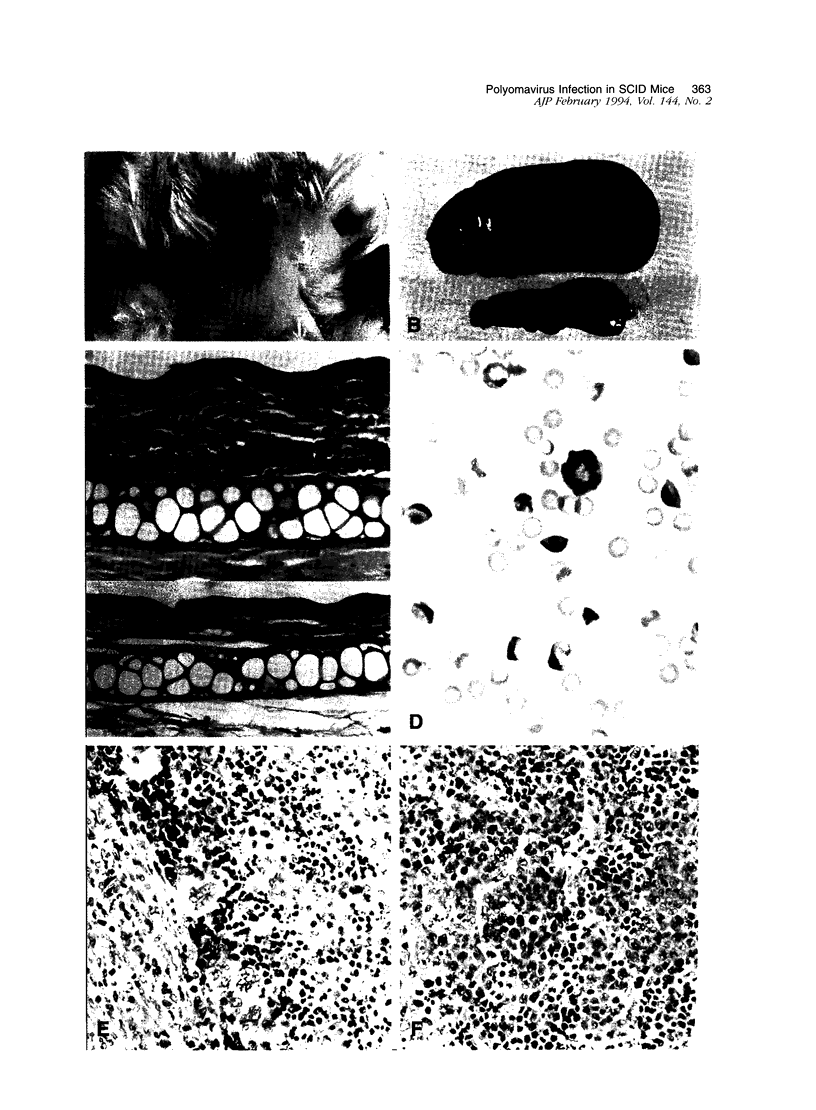
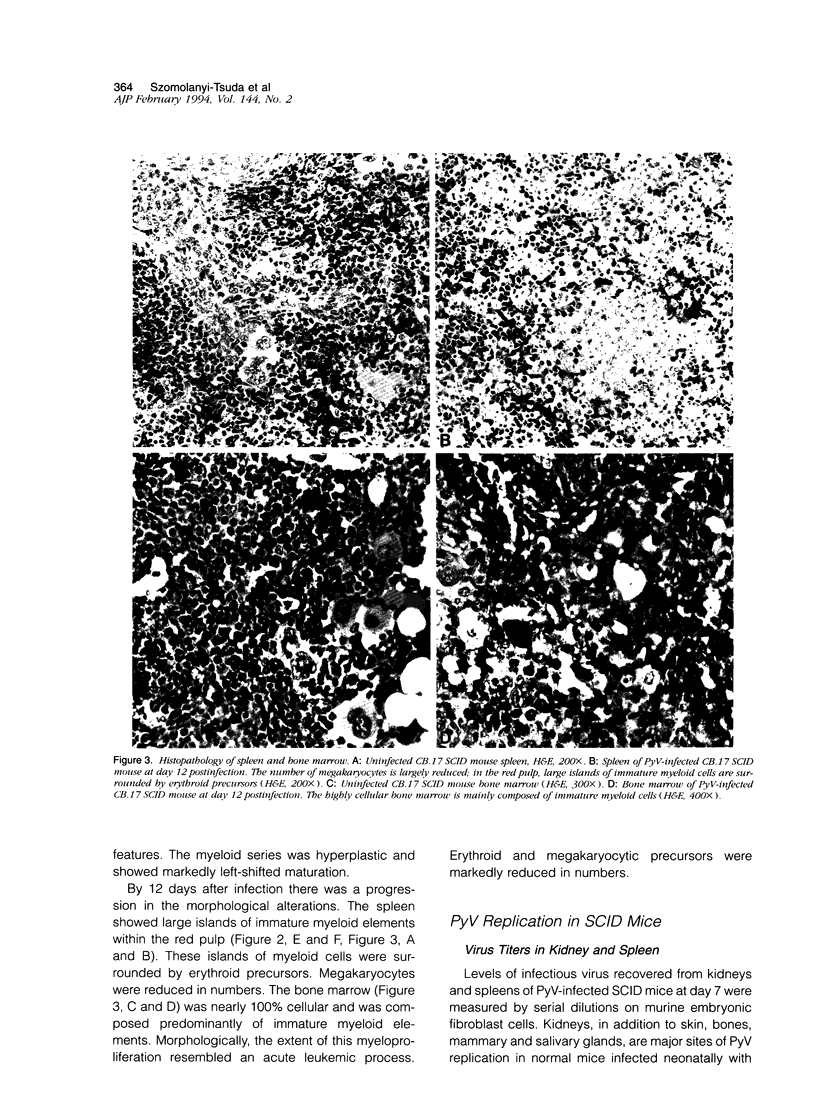
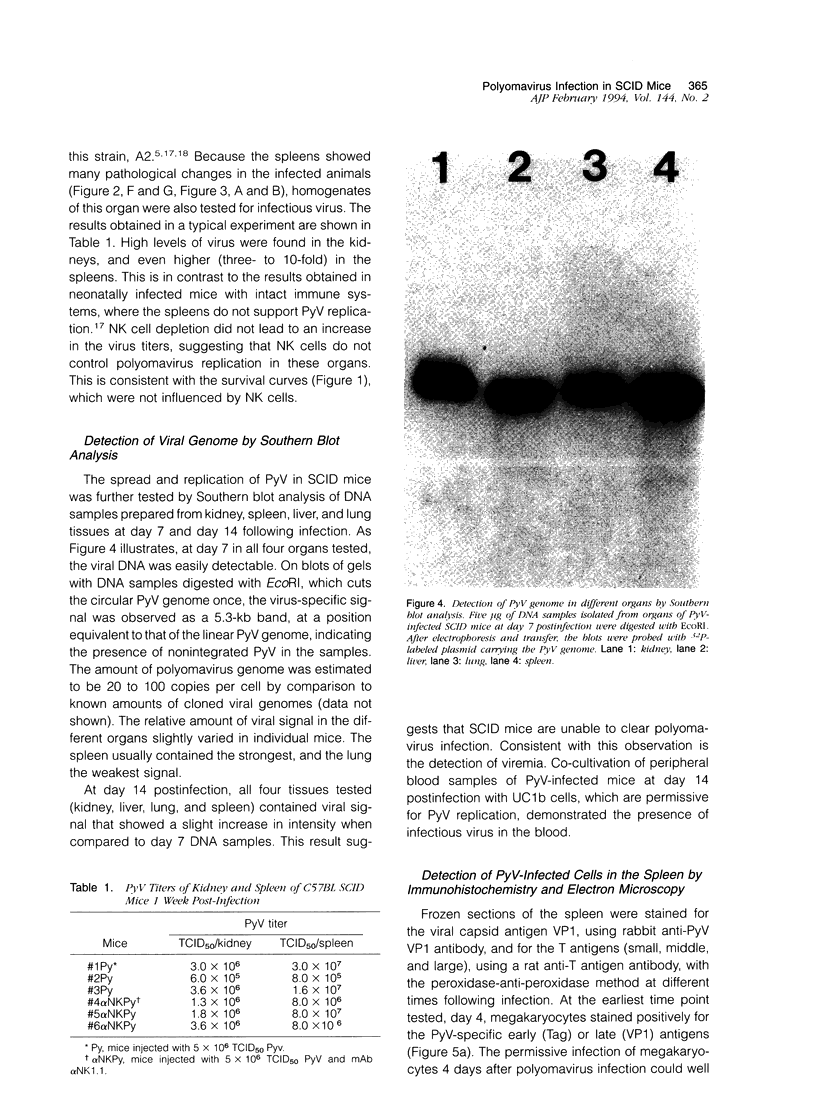
Infection of severe combined immunodeficient mice, which lack T and B lymphocytes, with polyomavirus (PyV) induced an acute hematological disorder leading to the death of the mice by 2 weeks postinfection. The disease was characterized by a dramatic decrease in megakaryocytes, multiple hemorrhages, anemia, thrombocytopenia, splenomegaly, a massive myeloproliferation and splenic erythroproliferation with a defect in maturation of the myeloid elements similar to that in acute leukemia. This pathology in severe combined immunodeficient mice is very different from that of the well-characterized tumor profiles induced by PyV in normal newborn or nude mice. Viral T and capsid (VP1) antigens and viral genome were detected in some cells in the spleen, but not in the majority of the proliferating myeloid cells. This suggests that the myeloproliferation is induced by some indirect mechanism, such as secretion of growth factors or cytokines by virus-infected cells, rather than by direct transformation by PyV. Neither the spread of PyV, its replication in different organs, nor the pathogenesis or the time of death were altered by depleting natural killer cells in vivo by anti-natural killer cell antibodies. Analysis of the spleen leukocyte population indicated that the cells expressed high levels of class I major histocompatibility complex antigens and were resistant to lysis by activated natural killer cells.
Full text
PDF


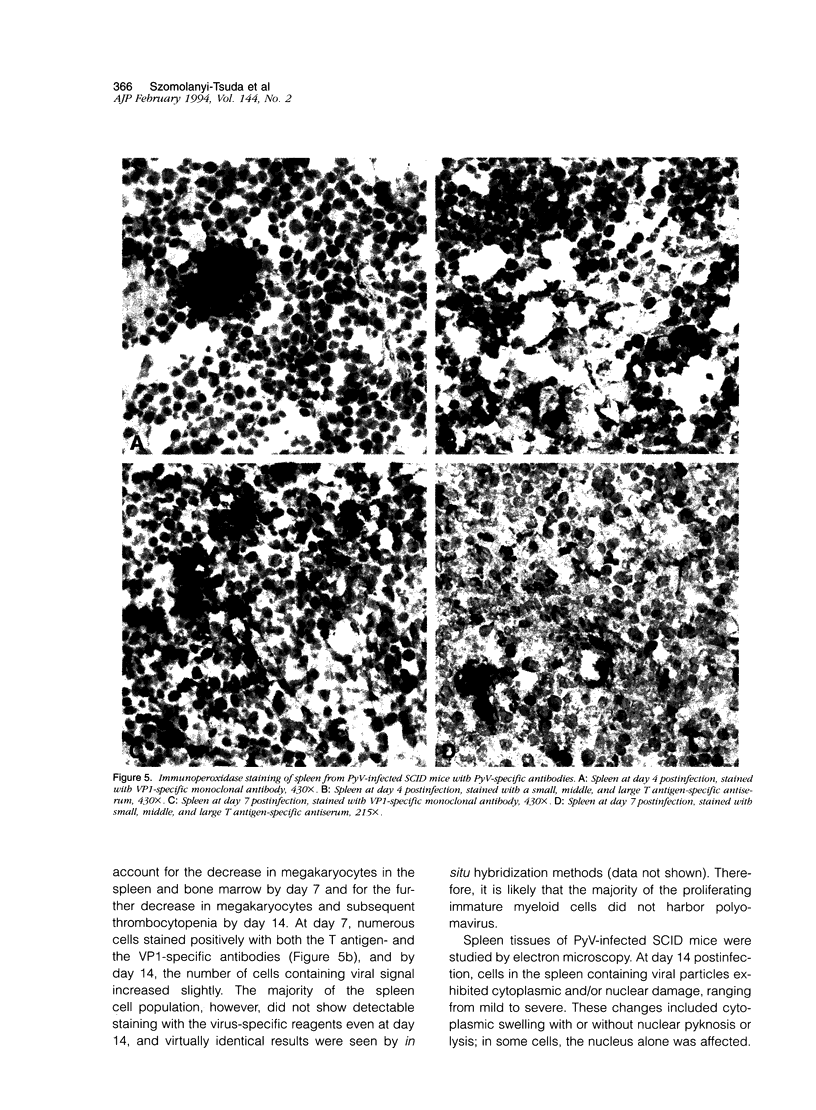
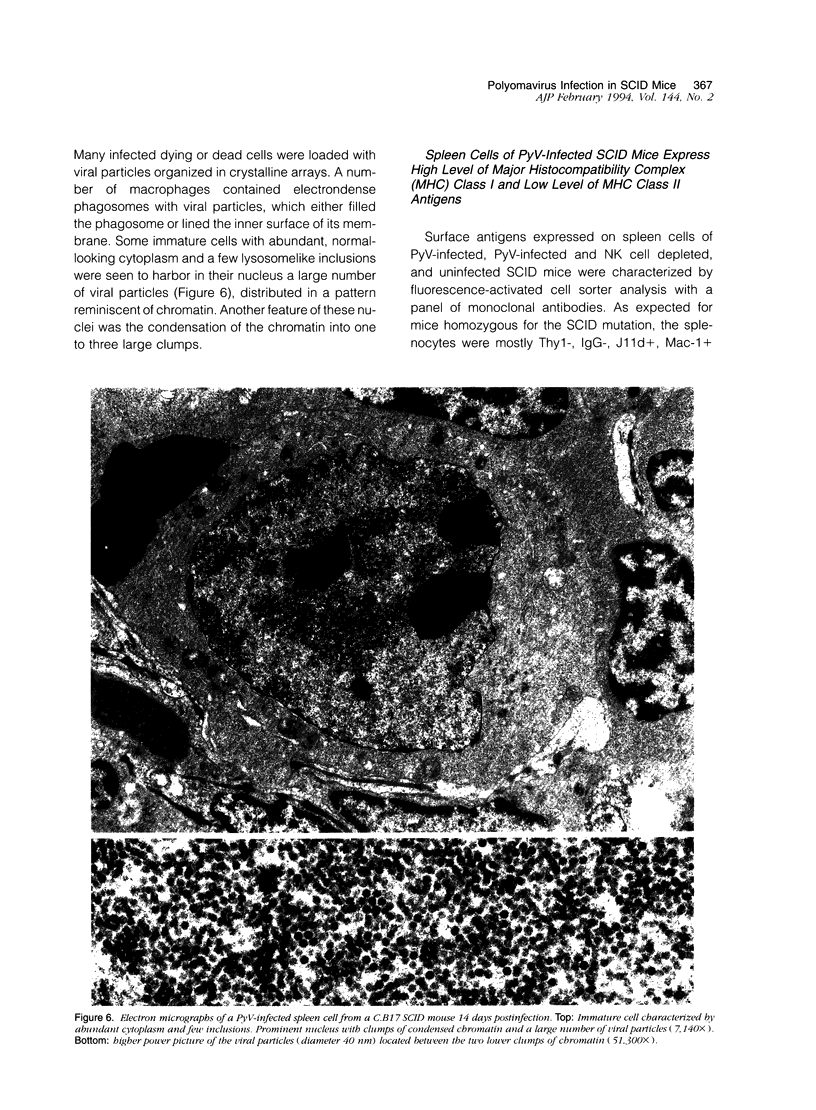
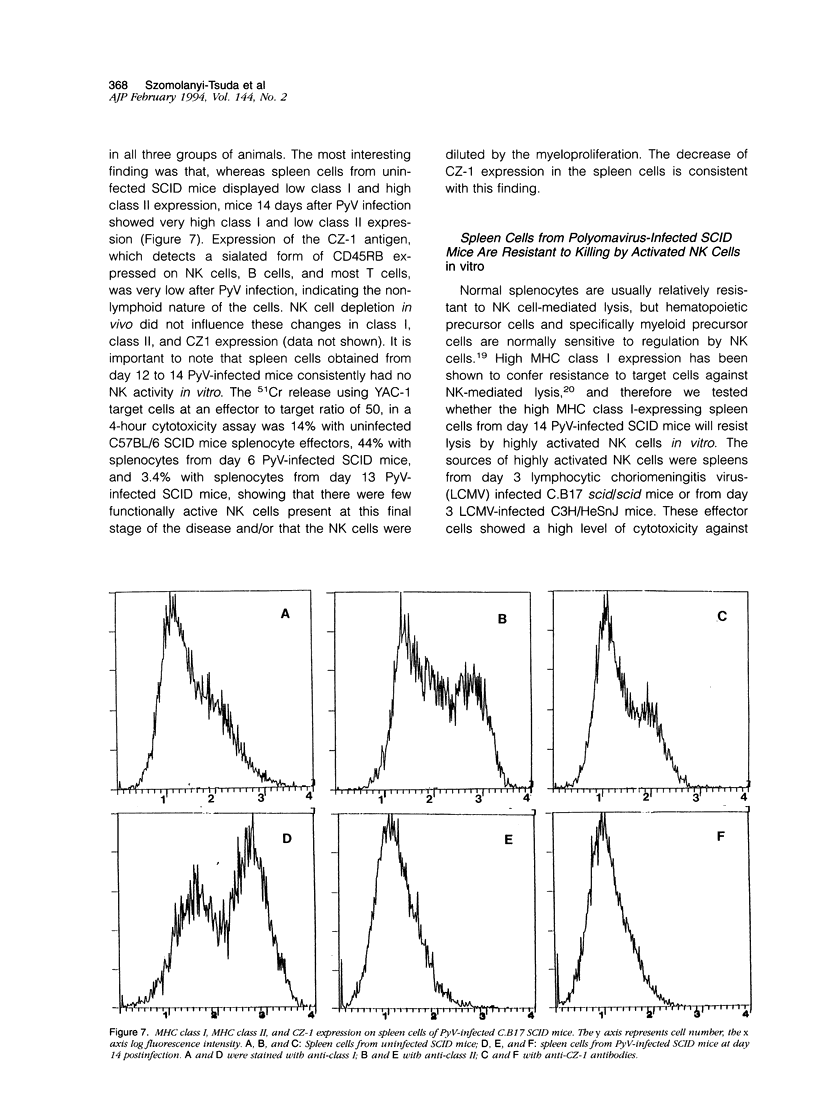
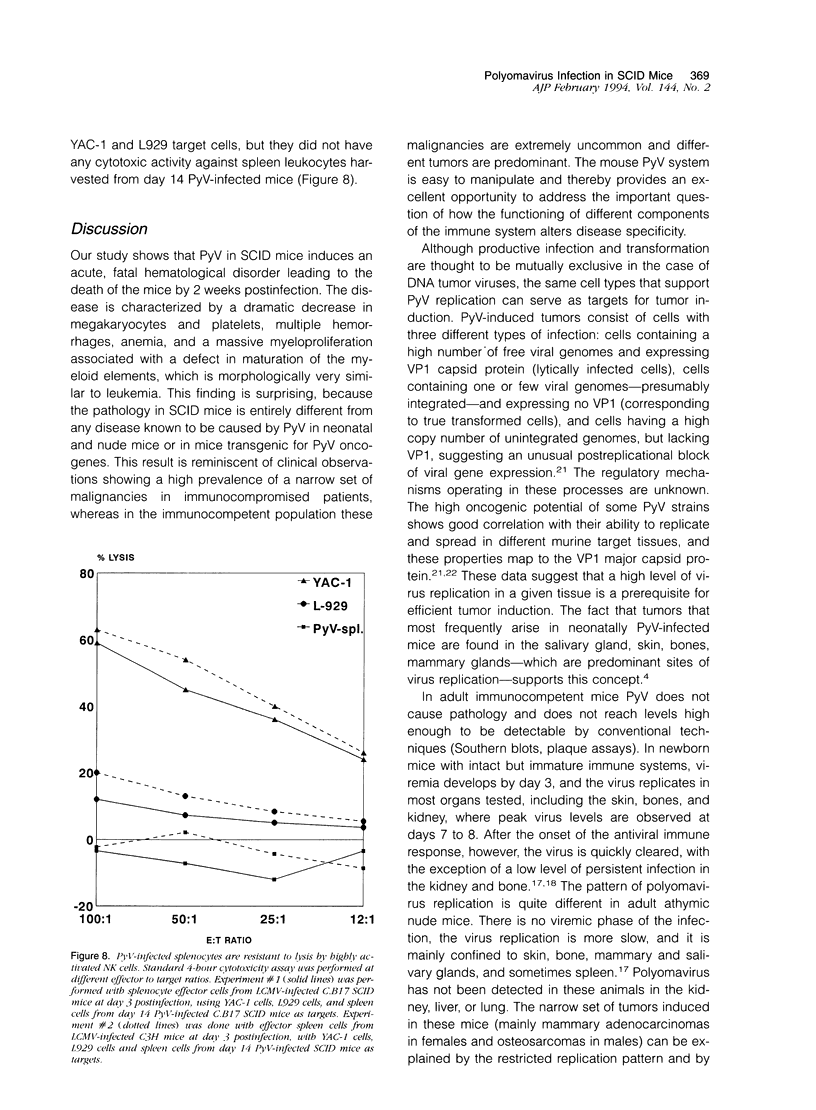


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bautch V. L., Toda S., Hassell J. A., Hanahan D. Endothelial cell tumors develop in transgenic mice carrying polyoma virus middle T oncogene. Cell. 1987 Nov 20;51(4):529–537. doi: 10.1016/0092-8674(87)90122-x. [DOI] [PubMed] [Google Scholar]
- Berebbi M., Dandolo L., Hassoun J., Bernard A. M., Blangy D. Specific tissue targeting of polyoma virus oncogenicity in athymic nude mice. Oncogene. 1988 Feb;2(2):149–156. [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Brutkiewicz R. R., O'Donnell C. L., Maciaszek J. W., Welsh R. M., Vargas-Cortes M. The monoclonal antibody CZ-1 identifies a mouse CD45-associated epitope expressed on interleukin-2-responsive cells. Eur J Immunol. 1993 Oct;23(10):2427–2433. doi: 10.1002/eji.1830231008. [DOI] [PubMed] [Google Scholar]
- Dawe C. J., Freund R., Abromson-Leeman S. R., Dubensky T. W., Carroll J., Dorf M. E., Benjamin T. L. T-cell lymphomas emerging as epineoplasms in mice bearing transplanted polyoma virus-induced salivary gland tumors. Cancer Res. 1990 Sep 1;50(17 Suppl):5643S–5648S. [PubMed] [Google Scholar]
- Dawe C. J., Freund R., Mandel G., Ballmer-Hofer K., Talmage D. A., Benjamin T. L. Variations in polyoma virus genotype in relation to tumor induction in mice. Characterization of wild type strains with widely differing tumor profiles. Am J Pathol. 1987 May;127(2):243–261. [PMC free article] [PubMed] [Google Scholar]
- Dubensky T. W., Freund R., Dawe C. J., Benjamin T. L. Polyomavirus replication in mice: influences of VP1 type and route of inoculation. J Virol. 1991 Jan;65(1):342–349. doi: 10.1128/jvi.65.1.342-349.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovich A. H., Mathur A., Kamat D., Shapiro R. S. Primary immunodeficiencies: genetic risk factors for lymphoma. Cancer Res. 1992 Oct 1;52(19 Suppl):5465s–5467s. [PubMed] [Google Scholar]
- Freund R., Calderone A., Dawe C. J., Benjamin T. L. Polyomavirus tumor induction in mice: effects of polymorphisms of VP1 and large T antigen. J Virol. 1991 Jan;65(1):335–341. doi: 10.1128/jvi.65.1.335-341.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy C. T., Cardiff R. D., Muller W. J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992 Mar;12(3):954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgy K. E., Burns G. F., Hayhoe F. G. Discrimination of B, T and null lymphocytes by esterase cytochemistry. Scand J Haematol. 1977 May;18(5):437–448. doi: 10.1111/j.1600-0609.1977.tb02098.x. [DOI] [PubMed] [Google Scholar]
- Hoover R. N. Lymphoma risks in populations with altered immunity--a search for mechanism. Cancer Res. 1992 Oct 1;52(19 Suppl):5477s–5478s. [PubMed] [Google Scholar]
- Ioachim H. L. The opportunistic tumors of immune deficiency. Adv Cancer Res. 1990;54:301–317. [PubMed] [Google Scholar]
- Koo G. C., Peppard J. R. Establishment of monoclonal anti-Nk-1.1 antibody. Hybridoma. 1984 Fall;3(3):301–303. doi: 10.1089/hyb.1984.3.301. [DOI] [PubMed] [Google Scholar]
- Kornbluth S., Cross F. R., Harbison M., Hanafusa H. Transformation of chicken embryo fibroblasts and tumor induction by the middle T antigen of polyomavirus carried in an avian retroviral vector. Mol Cell Biol. 1986 May;6(5):1545–1551. doi: 10.1128/mcb.6.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärre K. Natural killer cells and the MHC class I pathway of peptide presentation. Semin Immunol. 1993 Apr;5(2):127–145. doi: 10.1006/smim.1993.1016. [DOI] [PubMed] [Google Scholar]
- Medveczky P., Szomolanyi E., Desrosiers R. C., Mulder C. Classification of herpesvirus saimiri into three groups based on extreme variation in a DNA region required for oncogenicity. J Virol. 1984 Dec;52(3):938–944. doi: 10.1128/jvi.52.3.938-944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B., Herndier B., Meeker T., Kaplan L., McGrath M. Molecular and immunophenotypic characterization of AIDS-associated, Epstein-Barr virus-negative, polyclonal lymphoma. J Clin Oncol. 1992 Mar;10(3):383–389. doi: 10.1200/JCO.1992.10.3.383. [DOI] [PubMed] [Google Scholar]
- Talmage D. A., Freund R., Dubensky T., Salcedo M., Gariglio P., Rangel L. M., Dawe C. J., Benjamin T. L. Heterogeneity in state and expression of viral DNA in polyoma virus-induced tumors of the mouse. Virology. 1992 Apr;187(2):734–747. doi: 10.1016/0042-6822(92)90476-6. [DOI] [PubMed] [Google Scholar]
- Tutt M. M., Schuler W., Kuziel W. A., Tucker P. W., Bennett M., Bosma M. J., Kumar V. T cell receptor genes do not rearrange or express functional transcripts in natural killer cells of scid mice. J Immunol. 1987 Apr 1;138(7):2338–2344. [PubMed] [Google Scholar]
- Welsh R. M., Brubaker J. O., Vargas-Cortes M., O'Donnell C. L. Natural killer (NK) cell response to virus infections in mice with severe combined immunodeficiency. The stimulation of NK cells and the NK cell-dependent control of virus infections occur independently of T and B cell function. J Exp Med. 1991 May 1;173(5):1053–1063. doi: 10.1084/jem.173.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J Exp Med. 1978 Jul 1;148(1):163–181. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. L., Courtneidge S. A., Wagner E. F. Embryonic lethalities and endothelial tumors in chimeric mice expressing polyoma virus middle T oncogene. Cell. 1988 Jan 15;52(1):121–131. doi: 10.1016/0092-8674(88)90536-3. [DOI] [PubMed] [Google Scholar]
- Wirth J. J., Amalfitano A., Gross R., Oldstone M. B., Fluck M. M. Organ- and age-specific replication of polyomavirus in mice. J Virol. 1992 Jun;66(6):3278–3286. doi: 10.1128/jvi.66.6.3278-3286.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth J. J., Fluck M. M. Immunological elimination of infected cells as the candidate mechanism for tumor protection in polyomavirus-infected mice. J Virol. 1991 Dec;65(12):6985–6988. doi: 10.1128/jvi.65.12.6985-6988.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]