Abstract
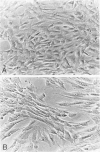
Pericytes are cells localized at the abluminal side of the microvascular endothelium and completely enveloped by a basement membrane. Pericytes have close contact with endothelial cells and are probably involved in the regulation of endothelial cell functions. Previous studies suggested a role for pericytes in microvascular proliferation in tumors. To study this cell type, we isolated human brain pericytes from microvessel segments derived from autopsy brain tissue. These cells were characterized in vitro using a panel of monoclonal antibodies. Human brain pericytes were reactive with monoclonal antibodies directed against the high molecular weight-melanoma associated antigen and intercellular adhesion molecule-1, but only a minority of the cells expressed alpha-smooth muscle actin (alpha-SMA, 0 to 10%) or vascular cell adhesion molecule-1 (10 to 50%). In histologically normal human brain microvessels in situ, pericytes consistently lacked staining for these four markers. Tissue with microvascular proliferation, however, showed a marked pericyte staining for both alpha-SMA and high molecular weight-melanoma associated antigen. The expression of alpha-SMA in vitro could be slightly up-regulated by incubation with serum-containing medium. An increase in alpha-SMA expression up to 40% of the total cell population was seen when pericytes were treated with transforming growth factor-beta 1, whereas basic fibroblast growth factor slightly inhibited alpha-SMA expression. Incubation with other factors (platelet-derived growth factor-AA, heparin, interferon-gamma, tumor necrosis factor-alpha) had no effect on the alpha-SMA expression at all. Transforming growth factor-beta 1 thus induces smooth muscle-like differentiation in pericytes in vitro and might play a role in the activation of pericytes during angiogenesis in vivo.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonelli-Orlidge A., Saunders K. B., Smith S. R., D'Amore P. A. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4544–4548. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciniegas E., Sutton A. B., Allen T. D., Schor A. M. Transforming growth factor beta 1 promotes the differentiation of endothelial cells into smooth muscle-like cells in vitro. J Cell Sci. 1992 Oct;103(Pt 2):521–529. doi: 10.1242/jcs.103.2.521. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Carlson E. C. Fenestrated subendothelial basement membranes in human retinal capillaries. Invest Ophthalmol Vis Sci. 1989 Sep;30(9):1923–1932. [PubMed] [Google Scholar]
- Crocker D. J., Murad T. M., Geer J. C. Role of the pericyte in wound healing. An ultrastructural study. Exp Mol Pathol. 1970 Aug;13(1):51–65. doi: 10.1016/0014-4800(70)90084-5. [DOI] [PubMed] [Google Scholar]
- Desmoulière A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993 Jul;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmoulière A., Rubbia-Brandt L., Abdiu A., Walz T., Macieira-Coelho A., Gabbiani G. Alpha-smooth muscle actin is expressed in a subpopulation of cultured and cloned fibroblasts and is modulated by gamma-interferon. Exp Cell Res. 1992 Jul;201(1):64–73. doi: 10.1016/0014-4827(92)90348-c. [DOI] [PubMed] [Google Scholar]
- Desmoulière A., Rubbia-Brandt L., Grau G., Gabbiani G. Heparin induces alpha-smooth muscle actin expression in cultured fibroblasts and in granulation tissue myofibroblasts. Lab Invest. 1992 Dec;67(6):716–726. [PubMed] [Google Scholar]
- Dorovini-Zis K., Prameya R., Bowman P. D. Culture and characterization of microvascular endothelial cells derived from human brain. Lab Invest. 1991 Mar;64(3):425–436. [PubMed] [Google Scholar]
- Díaz-Flores L., Gutiérrez R., Varela H., Rancel N., Valladares F. Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol. 1991 Apr;6(2):269–286. [PubMed] [Google Scholar]
- Elger M., Drenckhahn D., Nobiling R., Mundel P., Kriz W. Cultured rat mesangial cells contain smooth muscle alpha-actin not found in vivo. Am J Pathol. 1993 Feb;142(2):497–509. [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T., Singer S. J. Immunocytochemical studies of desmin and vimentin in pericapillary cells of chicken. J Histochem Cytochem. 1987 Oct;35(10):1105–1115. doi: 10.1177/35.10.3305702. [DOI] [PubMed] [Google Scholar]
- Haddad S. F., Moore S. A., Schelper R. L., Goeken J. A. Vascular smooth muscle hyperplasia underlies the formation of glomeruloid vascular structures of glioblastoma multiforme. J Neuropathol Exp Neurol. 1992 Sep;51(5):488–492. doi: 10.1097/00005072-199209000-00002. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Hellstrand M., Rymo L., Rubbia L., Gabbiani G. Interferon gamma inhibits both proliferation and expression of differentiation-specific alpha-smooth muscle actin in arterial smooth muscle cells. J Exp Med. 1989 Nov 1;170(5):1595–1608. doi: 10.1084/jem.170.5.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman I. M., D'Amore P. A. Microvascular pericytes contain muscle and nonmuscle actins. J Cell Biol. 1985 Jul;101(1):43–52. doi: 10.1083/jcb.101.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce N. C., DeCamilli P., Boyles J. Pericytes, like vascular smooth muscle cells, are immunocytochemically positive for cyclic GMP-dependent protein kinase. Microvasc Res. 1984 Sep;28(2):206–219. doi: 10.1016/0026-2862(84)90018-9. [DOI] [PubMed] [Google Scholar]
- Joyce N. C., Haire M. F., Palade G. E. Contractile proteins in pericytes. I. Immunoperoxidase localization of tropomyosin. J Cell Biol. 1985 May;100(5):1379–1386. doi: 10.1083/jcb.100.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce N. C., Haire M. F., Palade G. E. Contractile proteins in pericytes. II. Immunocytochemical evidence for the presence of two isomyosins in graded concentrations. J Cell Biol. 1985 May;100(5):1387–1395. doi: 10.1083/jcb.100.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley C., D'Amore P., Hechtman H. B., Shepro D. Microvascular pericyte contractility in vitro: comparison with other cells of the vascular wall. J Cell Biol. 1987 Mar;104(3):483–490. doi: 10.1083/jcb.104.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher O., Madri J. A. Modulation of actin mRNAs in cultured vascular cells by matrix components and TGF-beta 1. In Vitro Cell Dev Biol. 1989 May;25(5):424–434. doi: 10.1007/BF02624627. [DOI] [PubMed] [Google Scholar]
- Lehmann J. M., Holzmann B., Breitbart E. W., Schmiegelow P., Riethmüller G., Johnson J. P. Discrimination between benign and malignant cells of melanocytic lineage by two novel antigens, a glycoprotein with a molecular weight of 113,000 and a protein with a molecular weight of 76,000. Cancer Res. 1987 Feb 1;47(3):841–845. [PubMed] [Google Scholar]
- MOVAT H. Z., FERNANDO N. V. THE FINE STRUCTURE OF THE TERMINAL VASCULAR BED. IV. THE VENULES AND THEIR PERIVASCULAR CELLS (PERICYTES, ADVENTITIAL CELLS). Exp Mol Pathol. 1964 Apr;34:98–114. doi: 10.1016/0014-4800(64)90044-9. [DOI] [PubMed] [Google Scholar]
- Nehls V., Denzer K., Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992 Dec;270(3):469–474. doi: 10.1007/BF00645048. [DOI] [PubMed] [Google Scholar]
- Nehls V., Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol. 1991 Apr;113(1):147–154. doi: 10.1083/jcb.113.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehls V., Drenckhahn D. The versatility of microvascular pericytes: from mesenchyme to smooth muscle? Histochemistry. 1993 Jan;99(1):1–12. doi: 10.1007/BF00268014. [DOI] [PubMed] [Google Scholar]
- Nemes Z. Differentiation markers in hemangiopericytoma. Cancer. 1992 Jan 1;69(1):133–140. doi: 10.1002/1097-0142(19920101)69:1<133::aid-cncr2820690124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Orlidge A., D'Amore P. A. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987 Sep;105(3):1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M., Yang J., Buciak J., Tourtellotte W. W. Human brain microvascular DR-antigen. J Neurosci Res. 1989 Jul;23(3):337–341. doi: 10.1002/jnr.490230314. [DOI] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989 Jul;109(1):309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingemann R. O., Rietveld F. J., Kwaspen F., van de Kerkhof P. C., de Waal R. M., Ruiter D. J. Differential expression of markers for endothelial cells, pericytes, and basal lamina in the microvasculature of tumors and granulation tissue. Am J Pathol. 1991 Jun;138(6):1335–1347. [PMC free article] [PubMed] [Google Scholar]
- Schlingemann R. O., Rietveld F. J., de Waal R. M., Ferrone S., Ruiter D. J. Expression of the high molecular weight melanoma-associated antigen by pericytes during angiogenesis in tumors and in healing wounds. Am J Pathol. 1990 Jun;136(6):1393–1405. [PMC free article] [PubMed] [Google Scholar]
- Schürch W., Skalli O., Lagacé R., Seemayer T. A., Gabbiani G. Intermediate filament proteins and actin isoforms as markers for soft-tissue tumor differentiation and origin. III. Hemangiopericytomas and glomus tumors. Am J Pathol. 1990 Apr;136(4):771–786. [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Pelte M. F., Peclet M. C., Gabbiani G., Gugliotta P., Bussolati G., Ravazzola M., Orci L. Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J Histochem Cytochem. 1989 Mar;37(3):315–321. doi: 10.1177/37.3.2918221. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992 Dec;119(5):1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven D., Buyssens N. Desmin-positive stellate cells associated with angiogenesis in a tumour and non-tumour system. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;54(5):263–272. doi: 10.1007/BF02899222. [DOI] [PubMed] [Google Scholar]
- Wesseling P., Vandersteenhoven J. J., Downey B. T., Ruiter D. J., Burger P. C. Cellular components of microvascular proliferation in human glial and metastatic brain neoplasms. A light microscopic and immunohistochemical study of formalin-fixed, routinely processed material. Acta Neuropathol. 1993;85(5):508–514. doi: 10.1007/BF00230490. [DOI] [PubMed] [Google Scholar]
- Westphal J. R., Willems H. W., Schalkwijk C. J., Ruiter D. J., de Waal R. M. A new 180-kDa dermal endothelial cell activation antigen: in vitro and in situ characteristics. J Invest Dermatol. 1993 Jan;100(1):27–34. doi: 10.1111/1523-1747.ep12349946. [DOI] [PubMed] [Google Scholar]