Abstract
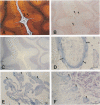
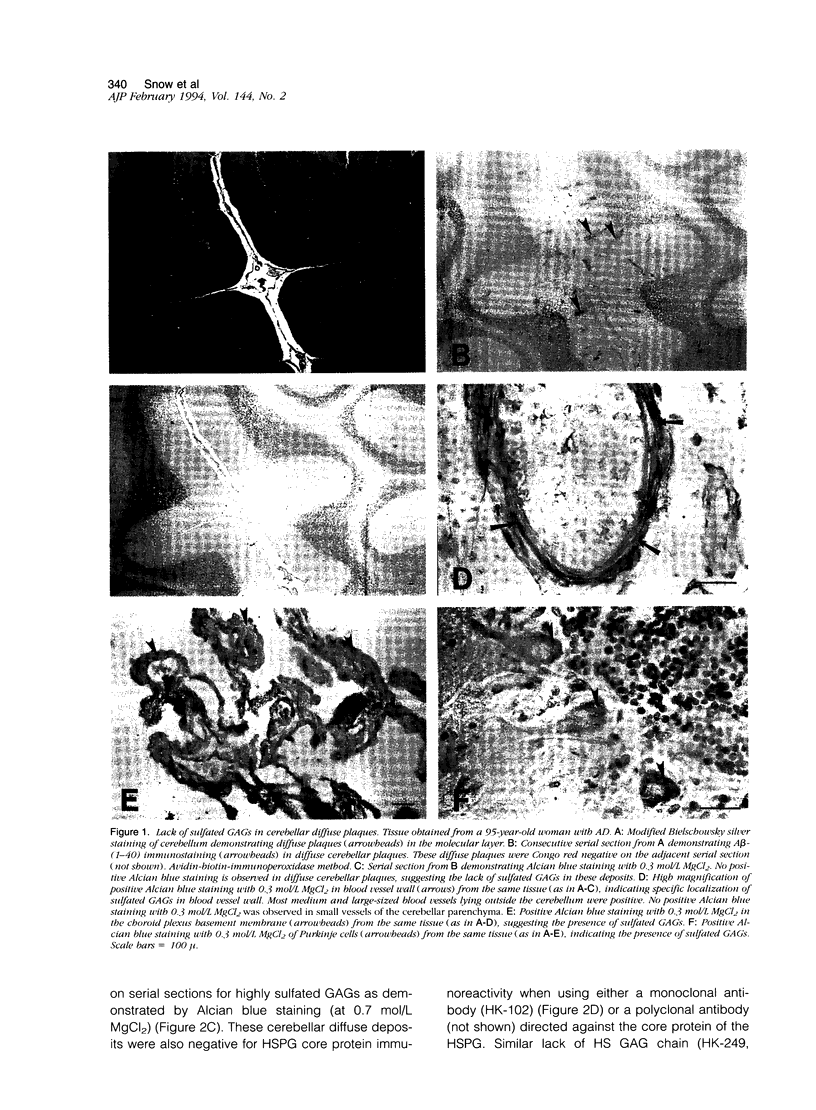
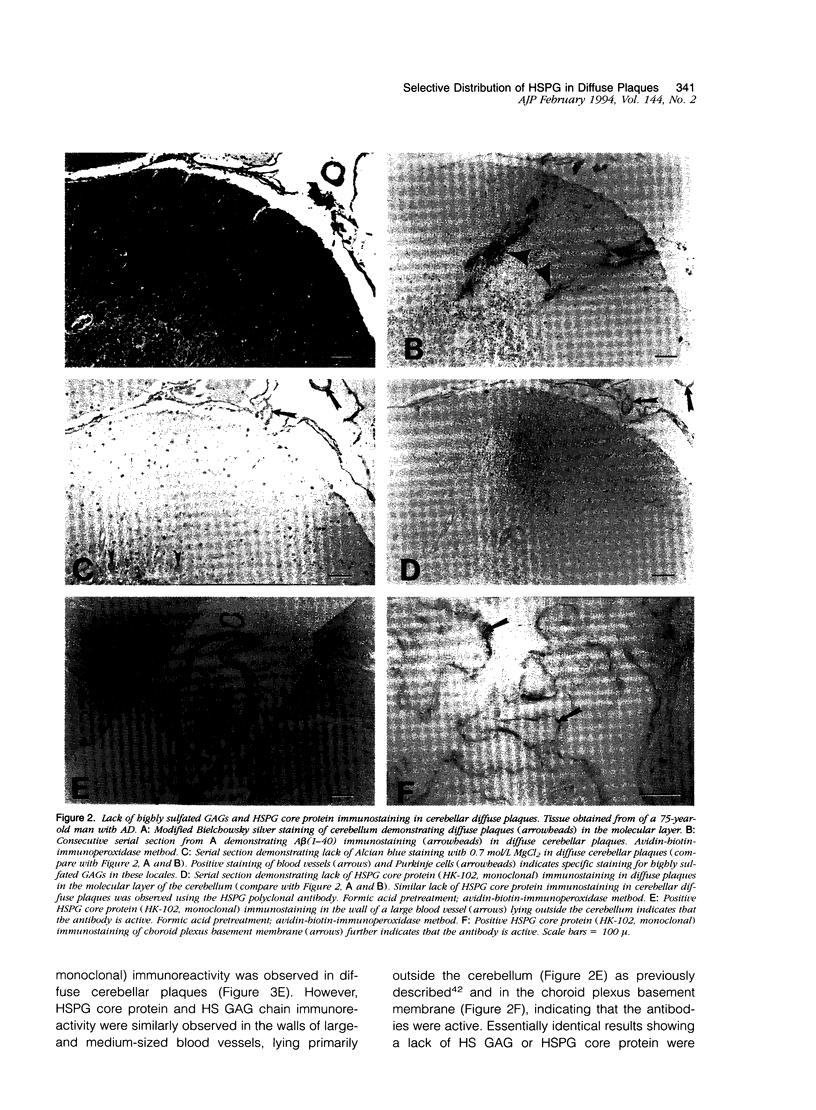
Previous studies have shown the basement membrane form of heparan sulfate proteoglycan (HSPG) known as perlecan, co-localized to beta-amyloid protein (A beta)-containing amyloid deposits in brains of patients with Alzheimer's disease (AD) and Down's syndrome. Although HSPG was localized to diffuse A beta plaques in hippocampus, amygdala, and neocortex, it is not known whether they are present in diffuse A beta plaques in cerebellum. In the present study, Alcian blue staining and immunocytochemical techniques were used to determine whether highly sulfated glycosaminoglycans (GAGs) and/or HSPG (perlecan) were also present in diffuse A beta plaques of cerebellum. Tissues from cases of AD were examined for the co-localization of highly sulfated GAGs, HSPGs, and A beta in diffuse plaques in cerebellum in comparison with hippocampus. Consecutive serial sections of AD brain tissue were stained or immunostained with 1) the modified Bielschowsky stain; 2) a polyclonal antibody directed against synthetic A beta (1-40); 3) Congo red; 4) Alcian blue (pH 5.7) with varying concentrations of magnesium chloride for identification of sulfated and highly sulfated GAGs; and 5) polyclonal and monoclonal antibodies recognizing either the core protein or a specific GAG epitope on perlecan. All cases (7 of 7) of AD contained diffuse A beta plaques in the cerebellum as identified by positive Bielschowsky staining and A beta immunoreactivity. None of these cases demonstrated positive Alcian blue staining (at 0.3 and 0.7 mol/L MgCl2), HSPG, or HS GAG immunoreactivity in the same diffuse cerebellar plaques on adjacent serial sections. However, Alcian blue staining, HSPG, and/or HS GAG immunoreactivity were observed in blood vessel walls, choroid plexus, and within Purkinje cells, suggesting that the techniques used were reliable and specific. In cerebellum, all plaques containing amyloid cores that were Congo red-positive were also positive for highly sulfated GAGs (by Alcian blue staining at 0.7 mol/L MgCl2) and HSPG (both core protein and GAG chain) immunoreactivity. Even though HSPG immunoreactivity was not present in cerebellar diffuse plaques, all cases (4 of 4) examined demonstrated HSPG (both core protein and GAG chain) immunoreactivity in diffuse A beta plaques in hippocampus. Therefore, by Alcian blue staining and immunocytochemical methods, highly sulfated GAGs and HSPGs are not present in A beta diffuse plaques in cerebellum. Since previous studies indicate that the cerebellum contains relatively few amyloid-containing plaques in comparison with diffuse plaques, these studies suggest that HSPG may be an essential component needed for amyloid formation and/or persistence in brain as observed in cortical areas.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham C. R., Selkoe D. J., Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988 Feb 26;52(4):487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Ard M. D., Bunge R. P. Heparan sulfate proteoglycan and laminin immunoreactivity on cultured astrocytes: relationship to differentiation and neurite growth. J Neurosci. 1988 Aug;8(8):2844–2858. doi: 10.1523/JNEUROSCI.08-08-02844.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyreuther K., Masters C. L. Nomenclature of amyloid A4 proteins and their precursors in Alzheimer's disease and Down's syndrome. Neurobiol Aging. 1990 Jan-Feb;11(1):66–68. doi: 10.1016/0197-4580(90)90067-a. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E., Bohl J., Lang W. Alzheimer's disease: amyloid plaques in the cerebellum. J Neurol Sci. 1989 Nov;93(2-3):277–287. doi: 10.1016/0022-510x(89)90197-4. [DOI] [PubMed] [Google Scholar]
- Cammarata S., Mancardi G., Tabaton M. Formic acid treatment exposes hidden neurofilament and tau epitopes in abnormal cytoskeletal filaments from patients with progressive supranuclear palsy and Alzheimer's disease. Neurosci Lett. 1990 Jul 31;115(2-3):351–355. doi: 10.1016/0304-3940(90)90481-n. [DOI] [PubMed] [Google Scholar]
- Caporaso G. L., Gandy S. E., Buxbaum J. D., Greengard P. Chloroquine inhibits intracellular degradation but not secretion of Alzheimer beta/A4 amyloid precursor protein. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2252–2256. doi: 10.1073/pnas.89.6.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. M., Huynh T. V., Saitoh T. Evidence for lysosomal processing of amyloid beta-protein precursor in cultured cells. Neurochem Res. 1989 Oct;14(10):933–939. doi: 10.1007/BF00965926. [DOI] [PubMed] [Google Scholar]
- Coria F., Castaño E., Prelli F., Larrondo-Lillo M., van Duinen S., Shelanski M. L., Frangione B. Isolation and characterization of amyloid P component from Alzheimer's disease and other types of cerebral amyloidosis. Lab Invest. 1988 Apr;58(4):454–458. [PubMed] [Google Scholar]
- Damon D. H., D'Amore P. A., Wagner J. A. Sulfated glycosaminoglycans modify growth factor-induced neurite outgrowth in PC12 cells. J Cell Physiol. 1988 May;135(2):293–300. doi: 10.1002/jcp.1041350217. [DOI] [PubMed] [Google Scholar]
- Dorling J. "Critical electrolyte concentration" method in histochemistry. J Med Lab Technol. 1969 Apr;26(2):124–130. [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Estus S., Golde T. E., Kunishita T., Blades D., Lowery D., Eisen M., Usiak M., Qu X. M., Tabira T., Greenberg B. D. Potentially amyloidogenic, carboxyl-terminal derivatives of the amyloid protein precursor. Science. 1992 Feb 7;255(5045):726–728. doi: 10.1126/science.1738846. [DOI] [PubMed] [Google Scholar]
- Fraser P. E., Nguyen J. T., Chin D. T., Kirschner D. A. Effects of sulfate ions on Alzheimer beta/A4 peptide assemblies: implications for amyloid fibril-proteoglycan interactions. J Neurochem. 1992 Oct;59(4):1531–1540. doi: 10.1111/j.1471-4159.1992.tb08470.x. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Golde T. E., Estus S., Younkin L. H., Selkoe D. J., Younkin S. G. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science. 1992 Feb 7;255(5045):728–730. doi: 10.1126/science.1738847. [DOI] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Hassell J. R., Robey P. G., Barrach H. J., Wilczek J., Rennard S. I., Martin G. R. Isolation of a heparan sulfate-containing proteoglycan from basement membrane. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4494–4498. doi: 10.1073/pnas.77.8.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holck M., Husby G., Sletten K., Natvig J. B. The amyloid P-component (protein AP): an integral part of the amyloid substance? Scand J Immunol. 1979;10(1):55–60. doi: 10.1111/j.1365-3083.1979.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Morris J. H., Selkoe D. J. Diffuse senile plaques occur commonly in the cerebellum in Alzheimer's disease. Am J Pathol. 1989 Aug;135(2):309–319. [PMC free article] [PubMed] [Google Scholar]
- Kalaria R. N., Galloway P. G., Perry G. Widespread serum amyloid P immunoreactivity in cortical amyloid deposits and the neurofibrillary pathology of Alzheimer's disease and other degenerative disorders. Neuropathol Appl Neurobiol. 1991 Jun;17(3):189–201. doi: 10.1111/j.1365-2990.1991.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Kalaria R. N., Perry G. Amyloid P component and other acute-phase proteins associated with cerebellar A beta-deposits in Alzheimer's disease. Brain Res. 1993 Dec 17;631(1):151–155. doi: 10.1016/0006-8993(93)91202-4. [DOI] [PubMed] [Google Scholar]
- Kato M., Koike Y., Suzuki S., Kimata K. Basement membrane proteoglycan in various tissues: characterization using monoclonal antibodies to the Engelbreth-Holm-Swarm mouse tumor low density heparan sulfate proteoglycan. J Cell Biol. 1988 Jun;106(6):2203–2210. doi: 10.1083/jcb.106.6.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh-Semba R., Oohira A., Kashiwamata S. Changes in glycosaminoglycans during the neuritogenesis in PC12 pheochromocytoma cells induced by nerve growth factor. J Neurochem. 1990 Nov;55(5):1749–1757. doi: 10.1111/j.1471-4159.1990.tb04965.x. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Ogomori K., Tateishi J., Prusiner S. B. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest. 1987 Aug;57(2):230–236. [PubMed] [Google Scholar]
- Margolis R. K., Salton S. R., Margolis R. U. Effects of nerve growth factor-induced differentiation on the heparan sulfate of PC12 pheochromocytoma cells and comparison with developing brain. Arch Biochem Biophys. 1987 Aug 15;257(1):107–114. doi: 10.1016/0003-9861(87)90548-0. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubbin W. D., Kay C. M., Narindrasorasak S., Kisilevsky R. Circular-dichroism studies on two murine serum amyloid A proteins. Biochem J. 1988 Dec 15;256(3):775–783. doi: 10.1042/bj2560775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomenclature of amyloid and amyloidosis. WHO-IUIS Nomenclature Sub-Committee. Bull World Health Organ. 1993;71(1):105–112. [PMC free article] [PubMed] [Google Scholar]
- Palmert M. R., Podlisny M. B., Witker D. S., Oltersdorf T., Younkin L. H., Selkoe D. J., Younkin S. G. The beta-amyloid protein precursor of Alzheimer disease has soluble derivatives found in human brain and cerebrospinal fluid. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6338–6342. doi: 10.1073/pnas.86.16.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmert M. R., Siedlak S. L., Podlisny M. B., Greenberg B., Shelton E. R., Chan H. W., Usiak M., Selkoe D. J., Perry G., Younkin S. G. Soluble derivatives of the beta amyloid protein precursor of Alzheimer's disease are labeled by antisera to the beta amyloid protein. Biochem Biophys Res Commun. 1989 Nov 30;165(1):182–188. doi: 10.1016/0006-291x(89)91052-8. [DOI] [PubMed] [Google Scholar]
- Perlmutter L. S., Chui H. C., Saperia D., Athanikar J. Microangiopathy and the colocalization of heparan sulfate proteoglycan with amyloid in senile plaques of Alzheimer's disease. Brain Res. 1990 Jan 29;508(1):13–19. doi: 10.1016/0006-8993(90)91111-s. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A., Olwin B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Riopelle R. J., Dow K. E. Functional interactions of neuronal heparan sulphate proteoglycans with laminin. Brain Res. 1990 Aug 13;525(1):92–100. doi: 10.1016/0006-8993(90)91324-a. [DOI] [PubMed] [Google Scholar]
- Rozemuller J. M., Stam F. C., Eikelenboom P. Acute phase proteins are present in amorphous plaques in the cerebral but not in the cerebellar cortex of patients with Alzheimer's disease. Neurosci Lett. 1990 Oct 30;119(1):75–78. doi: 10.1016/0304-3940(90)90759-3. [DOI] [PubMed] [Google Scholar]
- Rozemuller J. M., Stam F. C., Eikelenboom P. Acute phase proteins are present in amorphous plaques in the cerebral but not in the cerebellar cortex of patients with Alzheimer's disease. Neurosci Lett. 1990 Oct 30;119(1):75–78. doi: 10.1016/0304-3940(90)90759-3. [DOI] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M., Saitoh T., Cole G. Characterization of an amyloid beta precursor protein that binds heparin and contains tyrosine sulfate. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2066–2069. doi: 10.1073/pnas.86.6.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M. A., Mitchell M., Emerson D. L. Reconstituted basement membrane enhances neurite outgrowth in PC12 cells induced by nerve growth factor. Cell Growth Differ. 1990 Jul;1(7):313–318. [PubMed] [Google Scholar]
- Scott J. E., Dorling J. Differential staining of acid glycosaminoglycans (mucopolysaccharides) by alcian blue in salt solutions. Histochemie. 1965 Oct 1;5(3):221–233. doi: 10.1007/BF00306130. [DOI] [PubMed] [Google Scholar]
- Shoji M., Hirai S., Yamaguchi H., Harigaya Y., Ishiguro K., Matsubara E. Alpha 1-antichymotrypsin is present in diffuse senile plaques. A comparative study of beta-protein and alpha 1-antichymotrypsin immunostaining in the Alzheimer brain. Am J Pathol. 1991 Jan;138(1):247–257. [PMC free article] [PubMed] [Google Scholar]
- Sisodia S. S., Koo E. H., Beyreuther K., Unterbeck A., Price D. L. Evidence that beta-amyloid protein in Alzheimer's disease is not derived by normal processing. Science. 1990 Apr 27;248(4954):492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Lara S., Nochlin D., Wight T. N. Cationic dyes reveal proteoglycans structurally integrated within the characteristic lesions of Alzheimer's disease. Acta Neuropathol. 1989;78(2):113–123. doi: 10.1007/BF00688198. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Mar H., Nochlin D., Kimata K., Kato M., Suzuki S., Hassell J., Wight T. N. The presence of heparan sulfate proteoglycans in the neuritic plaques and congophilic angiopathy in Alzheimer's disease. Am J Pathol. 1988 Dec;133(3):456–463. [PMC free article] [PubMed] [Google Scholar]
- Snow A. D., Mar H., Nochlin D., Sekiguchi R. T., Kimata K., Koike Y., Wight T. N. Early accumulation of heparan sulfate in neurons and in the beta-amyloid protein-containing lesions of Alzheimer's disease and Down's syndrome. Am J Pathol. 1990 Nov;137(5):1253–1270. [PMC free article] [PubMed] [Google Scholar]
- Snow A. D., Wight T. N., Nochlin D., Koike Y., Kimata K., DeArmond S. J., Prusiner S. B. Immunolocalization of heparan sulfate proteoglycans to the prion protein amyloid plaques of Gerstmann-Straussler syndrome, Creutzfeldt-Jakob disease and scrapie. Lab Invest. 1990 Nov;63(5):601–611. [PubMed] [Google Scholar]
- Snow A. D., Wight T. N. Proteoglycans in the pathogenesis of Alzheimer's disease and other amyloidoses. Neurobiol Aging. 1989 Sep-Oct;10(5):481–497. doi: 10.1016/0197-4580(89)90108-5. [DOI] [PubMed] [Google Scholar]
- Su J. H., Cummings B. J., Cotman C. W. Localization of heparan sulfate glycosaminoglycan and proteoglycan core protein in aged brain and Alzheimer's disease. Neuroscience. 1992 Dec;51(4):801–813. doi: 10.1016/0306-4522(92)90521-3. [DOI] [PubMed] [Google Scholar]
- Turnbull J. E., Fernig D. G., Ke Y., Wilkinson M. C., Gallagher J. T. Identification of the basic fibroblast growth factor binding sequence in fibroblast heparan sulfate. J Biol Chem. 1992 May 25;267(15):10337–10341. [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Wong C. W., Quaranta V., Glenner G. G. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Hirai S., Morimatsu M., Shoji M., Nakazato Y. Diffuse type of senile plaques in the cerebellum of Alzheimer-type dementia demonstrated by beta protein immunostain. Acta Neuropathol. 1989;77(3):314–319. doi: 10.1007/BF00687584. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Nakazato Y., Hirai S., Shoji M. Immunoelectron microscopic localization of amyloid beta protein in the diffuse plaques of Alzheimer-type dementia. Brain Res. 1990 Feb 5;508(2):320–324. doi: 10.1016/0006-8993(90)90416-9. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Nakazato Y., Shoji M., Takatama M., Hirai S. Ultrastructure of diffuse plaques in senile dementia of the Alzheimer type: comparison with primitive plaques. Acta Neuropathol. 1991;82(1):13–20. doi: 10.1007/BF00310918. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Hirano A. A comparative study of modified Bielschowsky, Bodian and thioflavin S stains on Alzheimer's neurofibrillary tangles. Neuropathol Appl Neurobiol. 1986 Jan-Feb;12(1):3–9. doi: 10.1111/j.1365-2990.1986.tb00677.x. [DOI] [PubMed] [Google Scholar]
- Young I. D., Willmer J. P., Kisilevsky R. The ultrastructural localization of sulfated proteoglycans is identical in the amyloids of Alzheimer's disease and AA, AL, senile cardiac and medullary carcinoma-associated amyloidosis. Acta Neuropathol. 1989;78(2):202–209. doi: 10.1007/BF00688210. [DOI] [PubMed] [Google Scholar]