Abstract
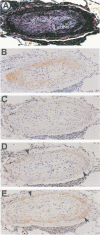
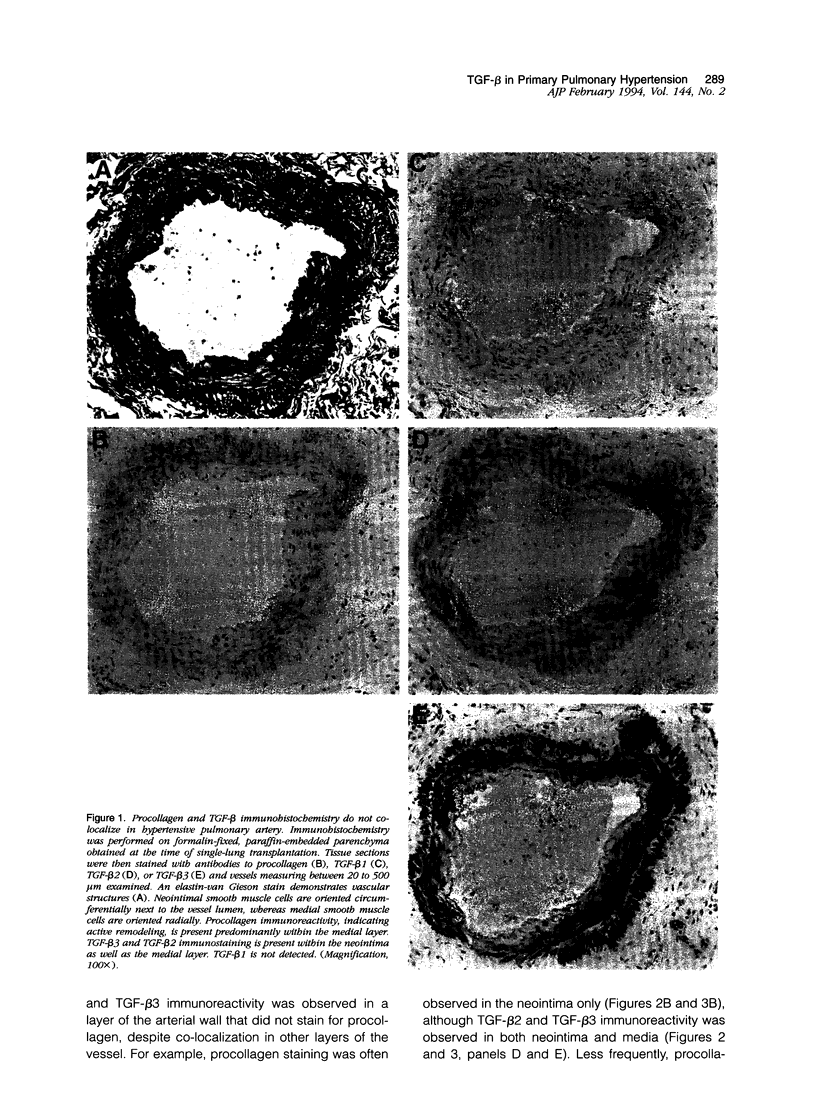
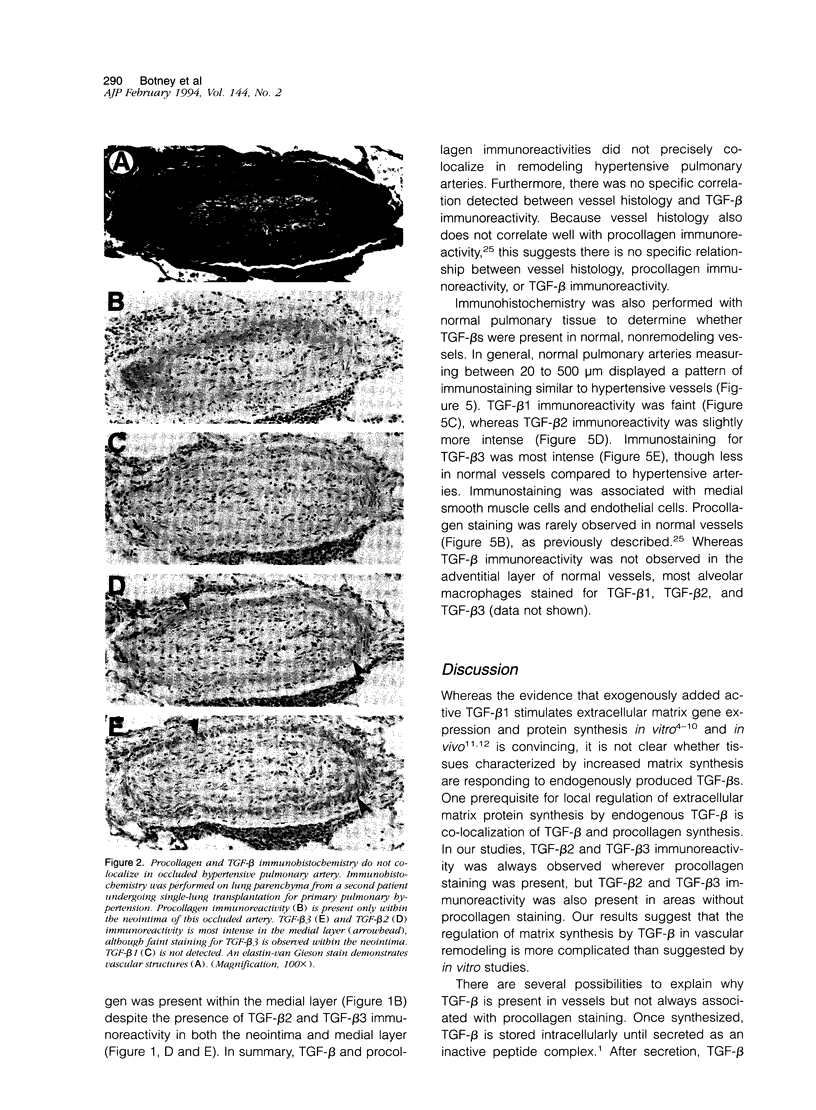
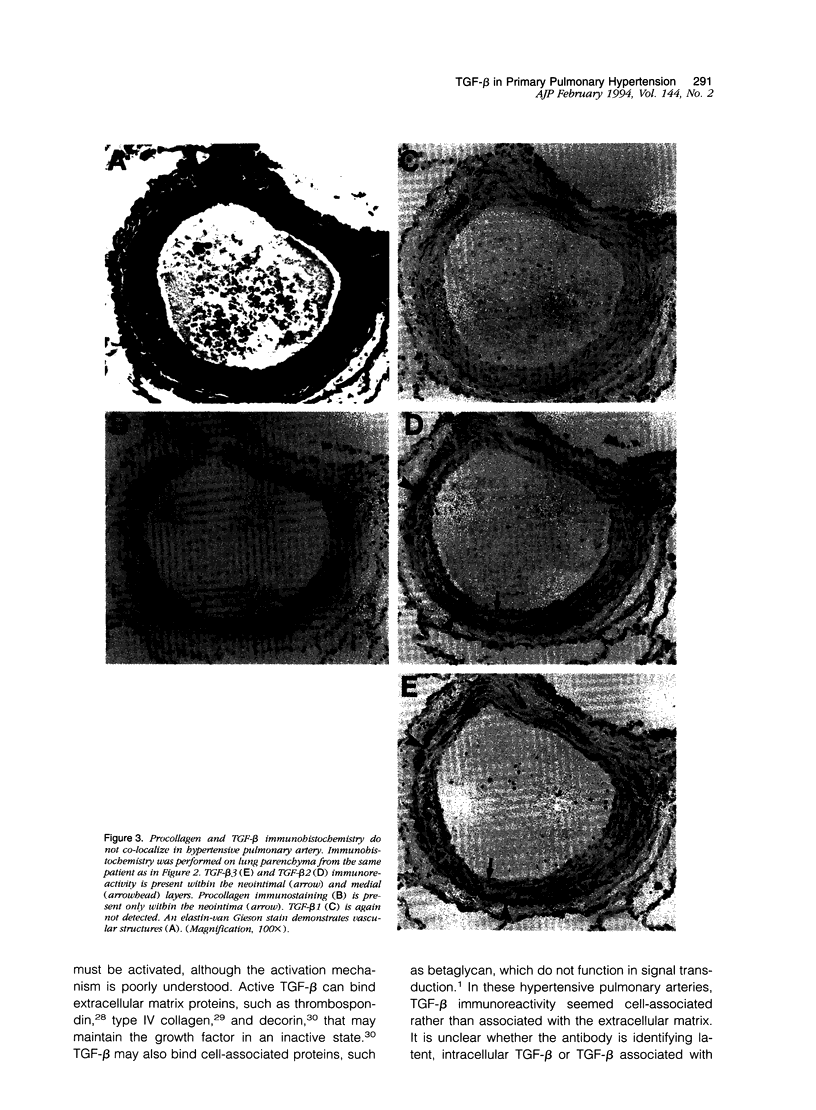
Active exogenous transforming growth factor-beta s (TGF-beta s) are potent modulators of extracellular matrix synthesis in cell culture and stimulate matrix synthesis in wounds and other remodeling tissues. The role of endogenous TGF-beta s in remodeling tissues is less well defined. Vascular remodeling in the pulmonary arteries of patients with primary pulmonary hypertension is characterized, in part, by abnormal deposition of immunohistochemically detectable procollagen, thereby identifying actively remodeling vessels. We used this marker of active matrix synthesis to begin defining the in vivo role of TGF-beta in the complex milieu of actively remodeling tissues. Immunohistochemistry using isoform-specific anti-TGF-beta antibodies was performed to determine whether TGF-beta was present in actively remodeling hypertensive pulmonary arteries 20 to 500 microns in diameter. Intense, cell-associated TGF-beta 3 immunoreactivity was observed in the media and neointima of these hypertensive muscular arteries. Immunostaining was present, but less intense, in normal arteries of comparable size. TGF-beta 2 immunoreactivity was observed in normal vessels and was increased slightly in hypertensive vessels, in a pattern resembling TGF-beta 3 immunoreactivity. No staining was associated with the adventitia. TGF-beta 1 immunostaining was either faint or absent in both normal and hypertensive vessels. Comparison of procollagen and TGF-beta localization demonstrated that TGF-beta 2 and TGF-beta 3 colocalized at all sites of procollagen synthesis. However, TGF-beta was observed in vessels, or vascular compartments, where there was no procollagen synthesis. Procollagen immunoreactivity was not present in normal vessels that showed immunoreactivity for TGF-beta 2 and TGF-beta 3. These observations suggest: a) the stimulation of procollagen synthesis by TGF-beta in vivo is more complex than suggested by in vitro studies and b) a potential role for TGF-beta 2 or TGF-beta 3, but not TGF-beta 1, in hypertensive pulmonary vascular remodeling.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Border W. A., Okuda S., Languino L. R., Sporn M. B., Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990 Jul 26;346(6282):371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- Border W. A., Okuda S., Languino L. R., Sporn M. B., Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990 Jul 26;346(6282):371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- Botney M. D., Liptay M. J., Kaiser L. R., Cooper J. D., Parks W. C., Mecham R. P. Active collagen synthesis by pulmonary arteries in human primary pulmonary hypertension. Am J Pathol. 1993 Jul;143(1):121–129. [PMC free article] [PubMed] [Google Scholar]
- Botney M. D., Parks W. C., Crouch E. C., Stenmark K., Mecham R. P. Transforming growth factor-beta 1 is decreased in remodeling hypertensive bovine pulmonary arteries. J Clin Invest. 1992 May;89(5):1629–1635. doi: 10.1172/JCI115759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekelmann T. J., Limper A. H., Colby T. V., McDonald J. A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. K., Hoshi H., McKeehan W. L. Transforming growth factor type beta specifically stimulates synthesis of proteoglycan in human adult arterial smooth muscle cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5287–5291. doi: 10.1073/pnas.84.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor T. B., Jr, Roberts A. B., Sporn M. B., Danielpour D., Dart L. L., Michels R. G., de Bustros S., Enger C., Kato H., Lansing M. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J Clin Invest. 1989 May;83(5):1661–1666. doi: 10.1172/JCI114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja M. J., Weiner F. R., Eghbali M., Giambrone M. A., Eghbali M., Zern M. A. Differential effects of gamma-interferon on collagen and fibronectin gene expression. J Biol Chem. 1987 Sep 25;262(27):13348–13351. [PubMed] [Google Scholar]
- Fine A., Goldstein R. H. The effect of transforming growth factor-beta on cell proliferation and collagen formation by lung fibroblasts. J Biol Chem. 1987 Mar 15;262(8):3897–3902. [PubMed] [Google Scholar]
- Gatherer D., Ten Dijke P., Baird D. T., Akhurst R. J. Expression of TGF-beta isoforms during first trimester human embryogenesis. Development. 1990 Oct;110(2):445–460. doi: 10.1242/dev.110.2.445. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Endo T., Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987 May 15;262(14):6443–6446. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Khalil N., Bereznay O., Sporn M., Greenberg A. H. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989 Sep 1;170(3):727–737. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J., Glick A., Sporn M. B., Roberts A. B. Characterization of the promoter region of the human transforming growth factor-beta 1 gene. J Biol Chem. 1989 Jan 5;264(1):402–408. [PubMed] [Google Scholar]
- Kuhn C., 3rd, Boldt J., King T. E., Jr, Crouch E., Vartio T., McDonald J. A. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis. 1989 Dec;140(6):1693–1703. doi: 10.1164/ajrccm/140.6.1693. [DOI] [PubMed] [Google Scholar]
- Kulozik M., Hogg A., Lankat-Buttgereit B., Krieg T. Co-localization of transforming growth factor beta 2 with alpha 1(I) procollagen mRNA in tissue sections of patients with systemic sclerosis. J Clin Invest. 1990 Sep;86(3):917–922. doi: 10.1172/JCI114793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafyatis R., Lechleider R., Kim S. J., Jakowlew S., Roberts A. B., Sporn M. B. Structural and functional characterization of the transforming growth factor beta 3 promoter. A cAMP-responsive element regulates basal and induced transcription. J Biol Chem. 1990 Nov 5;265(31):19128–19136. [PubMed] [Google Scholar]
- Levine J. H., Moses H. L., Gold L. I., Nanney L. B. Spatial and temporal patterns of immunoreactive transforming growth factor beta 1, beta 2, and beta 3 during excisional wound repair. Am J Pathol. 1993 Aug;143(2):368–380. [PMC free article] [PubMed] [Google Scholar]
- Liu J. M., Davidson J. M. The elastogenic effect of recombinant transforming growth factor-beta on porcine aortic smooth muscle cells. Biochem Biophys Res Commun. 1988 Aug 15;154(3):895–901. doi: 10.1016/0006-291x(88)90224-0. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Lindner V., Twardzik D. R., Schwartz S. M., Reidy M. A. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991 Sep;88(3):904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- McGowan S. E., McNamer R. Transforming growth factor-beta increases elastin production by neonatal rat lung fibroblasts. Am J Respir Cell Mol Biol. 1990 Oct;3(4):369–376. doi: 10.1165/ajrcmb/3.4.369. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Schultz-Cherry S., Hök M. Transforming growth factor-beta complexes with thrombospondin. Mol Biol Cell. 1992 Feb;3(2):181–188. doi: 10.1091/mbc.3.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma T., Glick A. B., Geiser A. G., O'Reilly M. A., Miller J., Roberts A. B., Sporn M. B. Molecular cloning and structure of the human transforming growth factor-beta 2 gene promoter. Growth Factors. 1991;4(4):247–255. doi: 10.3109/08977199109043910. [DOI] [PubMed] [Google Scholar]
- Paralkar V. M., Vukicevic S., Reddi A. H. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev Biol. 1991 Feb;143(2):303–308. doi: 10.1016/0012-1606(91)90081-d. [DOI] [PubMed] [Google Scholar]
- Pelton R. W., Dickinson M. E., Moses H. L., Hogan B. L. In situ hybridization analysis of TGF beta 3 RNA expression during mouse development: comparative studies with TGF beta 1 and beta 2. Development. 1990 Oct;110(2):609–620. doi: 10.1242/dev.110.2.609. [DOI] [PubMed] [Google Scholar]
- Pelton R. W., Saxena B., Jones M., Moses H. L., Gold L. I. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991 Nov;115(4):1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttinen R. P., Kobayashi S., Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkett E. A., Badesch D. B., Roessler M. K., Stenmark K. R., Meyrick B. Insulin-like growth factor I and pulmonary hypertension induced by continuous air embolization in sheep. Am J Respir Cell Mol Biol. 1992 Jan;6(1):82–87. doi: 10.1165/ajrcmb/6.1.82. [DOI] [PubMed] [Google Scholar]
- Perkett E. A., Lyons R. M., Moses H. L., Brigham K. L., Meyrick B. Transforming growth factor-beta activity in sheep lung lymph during the development of pulmonary hypertension. J Clin Invest. 1990 Nov;86(5):1459–1464. doi: 10.1172/JCI114862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Griffin G. L., Senior R. M., Deuel T. F. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989 Jul;109(1):429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietra G. G., Edwards W. D., Kay J. M., Rich S., Kernis J., Schloo B., Ayres S. M., Bergofsky E. H., Brundage B. H., Detre K. M. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation. 1989 Nov;80(5):1198–1206. doi: 10.1161/01.cir.80.5.1198. [DOI] [PubMed] [Google Scholar]
- Prosser I. W., Stenmark K. R., Suthar M., Crouch E. C., Mecham R. P., Parks W. C. Regional heterogeneity of elastin and collagen gene expression in intralobar arteries in response to hypoxic pulmonary hypertension as demonstrated by in situ hybridization. Am J Pathol. 1989 Dec;135(6):1073–1088. [PMC free article] [PubMed] [Google Scholar]
- Raghow B., Irish P., Kang A. H. Coordinate regulation of transforming growth factor beta gene expression and cell proliferation in hamster lungs undergoing bleomycin-induced pulmonary fibrosis. J Clin Invest. 1989 Dec;84(6):1836–1842. doi: 10.1172/JCI114369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Kim S. J., Noma T., Glick A. B., Lafyatis R., Lechleider R., Jakowlew S. B., Geiser A., O'Reilly M. A., Danielpour D. Multiple forms of TGF-beta: distinct promoters and differential expression. Ciba Found Symp. 1991;157:7–28. doi: 10.1002/9780470514061.ch2. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]