Abstract
We immunohistochemically studied the location of abnormal prion protein in the central nervous system and visceral organs at the clinical and preclinical stages of mice infected with Creutzfeldt-Jakob disease via intraperitoneal route. Abnormal prion protein was diffusely distributed in the central nervous system. The sequential study showed that its stainings were first detected 120 days after inoculation, were found in all mice after 180 days, and were the most intense and widespread after 270 days. There was no restricted involvement at the early stages nor rostrally dominant distribution of the stainings that had been found in mice infected via intracerebral route. Abnormal prion protein was also located in the follicular dendritic cells in the spleen, lymph nodes, intestinal Peyer's patch, and thymus. Its stainings were first detected in the spleen, lymph nodes, and Peyer's patch 14 or 30 days after inoculation. In the thymus, however, the stainings were first detected after 210 days in the germinal centers formed in the medulla.
Full text
PDF


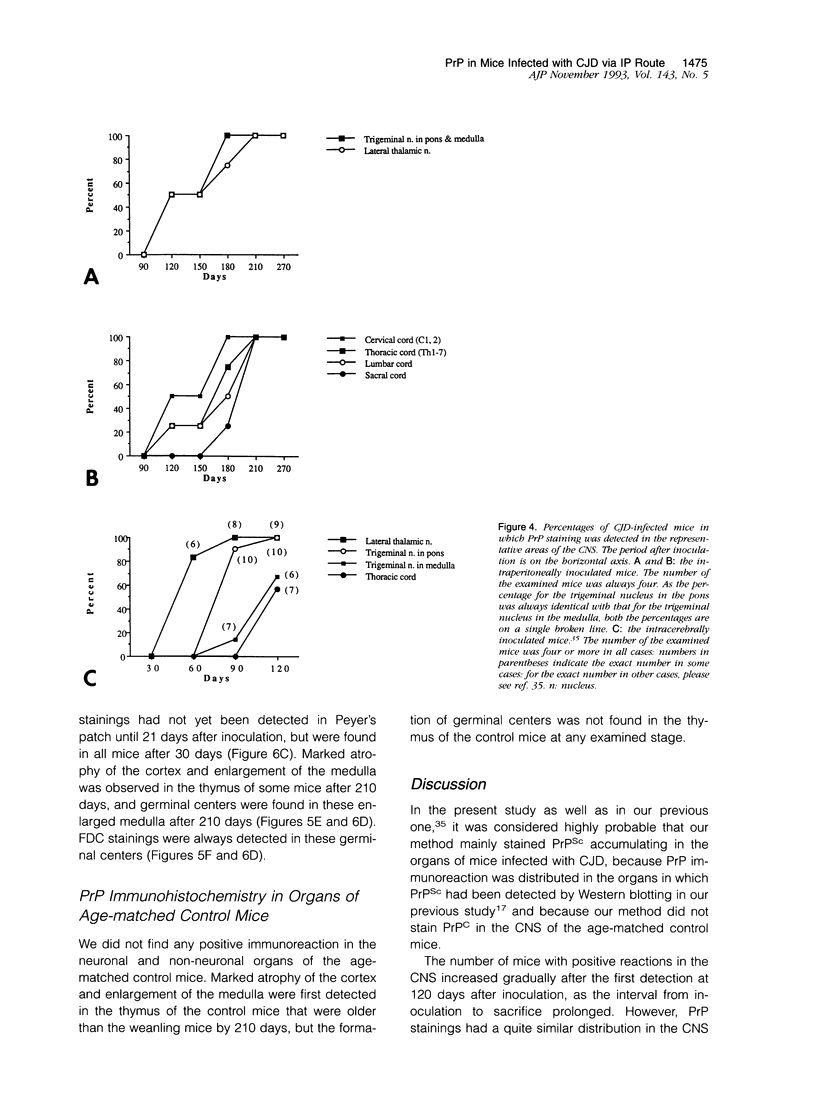
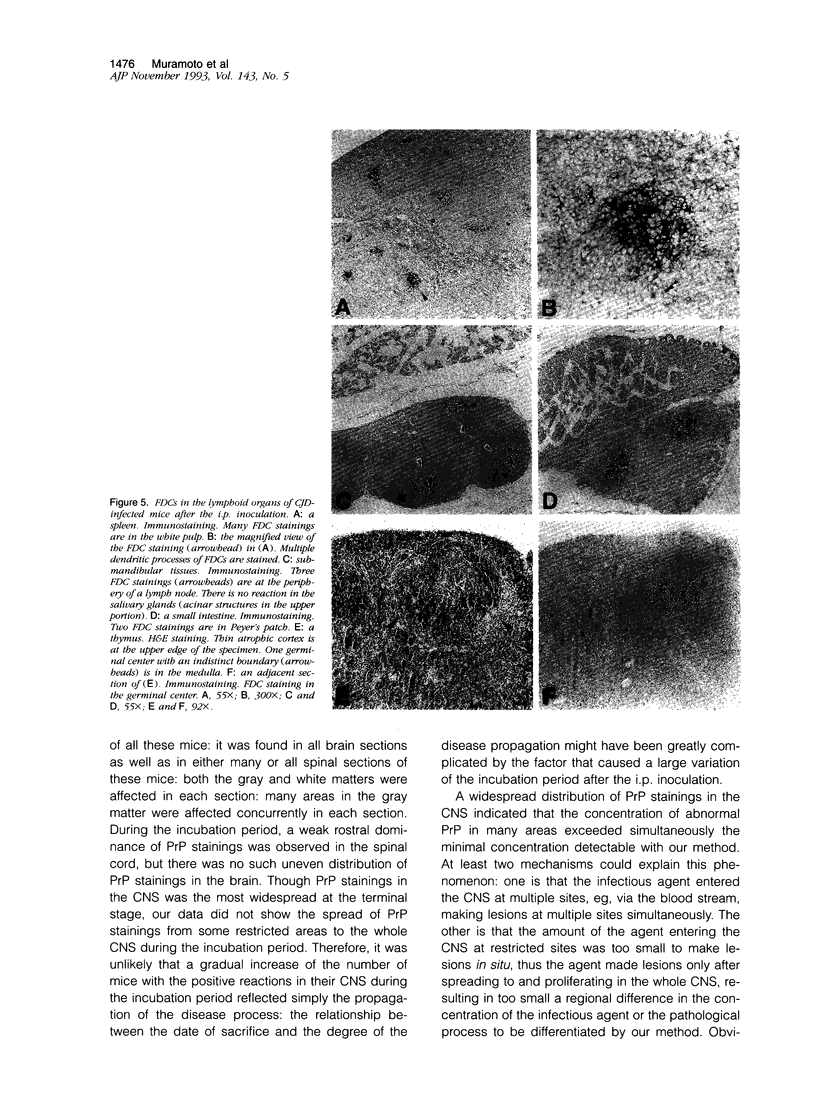
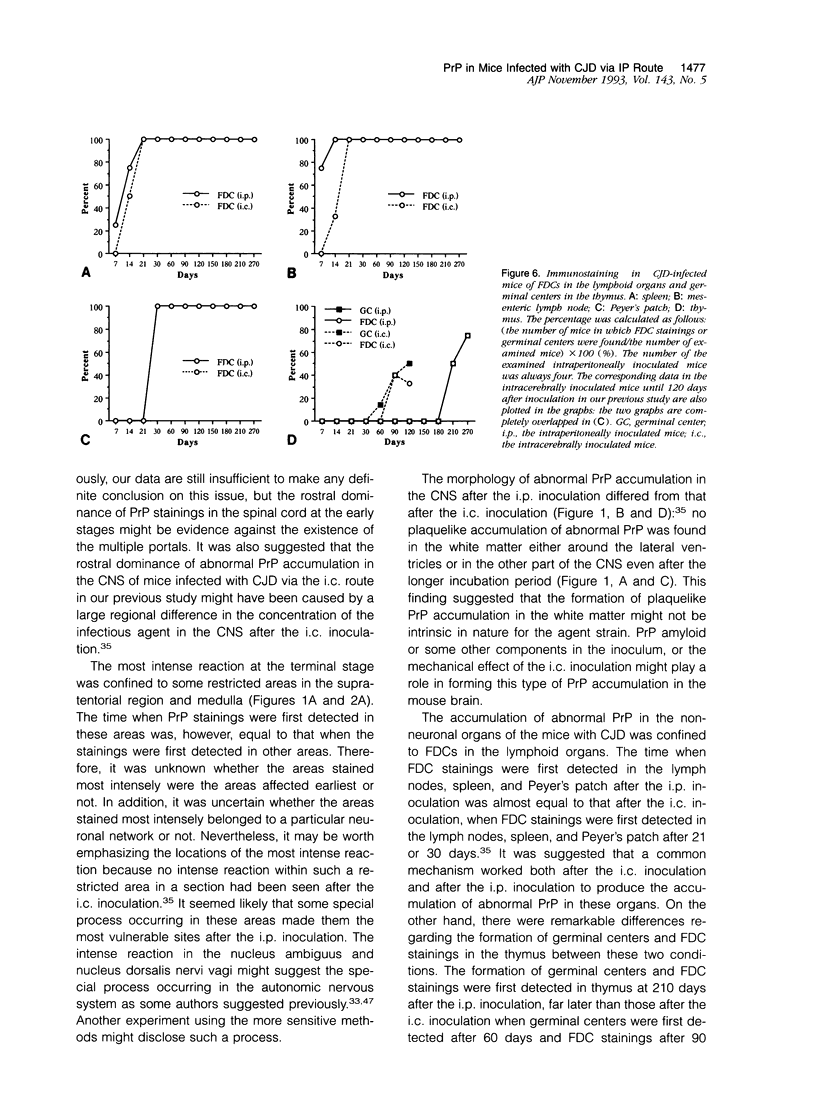


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry R. A., Prusiner S. B. Monoclonal antibodies to the cellular and scrapie prion proteins. J Infect Dis. 1986 Sep;154(3):518–521. doi: 10.1093/infdis/154.3.518. [DOI] [PubMed] [Google Scholar]
- Bockman J. M., Kingsbury D. T., McKinley M. P., Bendheim P. E., Prusiner S. B. Creutzfeldt-Jakob disease prion proteins in human brains. N Engl J Med. 1985 Jan 10;312(2):73–78. doi: 10.1056/NEJM198501103120202. [DOI] [PubMed] [Google Scholar]
- Brown P., Coker-Vann M., Pomeroy K., Franko M., Asher D. M., Gibbs C. J., Jr, Gajdusek D. C. Diagnosis of Creutzfeldt-Jakob disease by Western blot identification of marker protein in human brain tissue. N Engl J Med. 1986 Feb 27;314(9):547–551. doi: 10.1056/NEJM198602273140904. [DOI] [PubMed] [Google Scholar]
- CHANDLER R. L. Encephalopathy in mice produced by inoculation with scrapie brain material. Lancet. 1961 Jun 24;1(7191):1378–1379. doi: 10.1016/s0140-6736(61)92008-6. [DOI] [PubMed] [Google Scholar]
- Chen L. L., Adams J. C., Steinman R. M. Anatomy of germinal centers in mouse spleen, with special reference to "follicular dendritic cells". J Cell Biol. 1978 Apr;77(1):148–164. doi: 10.1083/jcb.77.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S., Kimberlin R. H. Pathogenesis of mouse scrapie: dynamics of vacuolation in brain and spinal cord after intraperitoneal infection. Neuropathol Appl Neurobiol. 1985 May-Jun;11(3):213–227. doi: 10.1111/j.1365-2990.1985.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Dickinson A. G., Outram G. W. Differences in access into the central nervous system of ME7 scrapie agent from two strains of mice. J Comp Pathol. 1973 Jan;83(1):13–18. doi: 10.1016/0021-9975(73)90022-4. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C., Gibbs C. J., Alpers M. Experimental transmission of a Kuru-like syndrome to chimpanzees. Nature. 1966 Feb 19;209(5025):794–796. doi: 10.1038/209794a0. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C. Unconventional viruses and the origin and disappearance of kuru. Science. 1977 Sep 2;197(4307):943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- Gibbs C. J., Jr, Gajdusek D. C., Asher D. M., Alpers M. P., Beck E., Daniel P. M., Matthews W. B. Creutzfeldt-Jakob disease (spongiform encephalopathy): transmission to the chimpanzee. Science. 1968 Jul 26;161(3839):388–389. doi: 10.1126/science.161.3839.388. [DOI] [PubMed] [Google Scholar]
- Hadlow W. J., Karstad L. Transmissible encephalopathy of mink in Ontario. Can Vet J. 1968 Aug;9(8):193–196. [PMC free article] [PubMed] [Google Scholar]
- Hadlow W. J., Kennedy R. C., Race R. E. Natural infection of Suffolk sheep with scrapie virus. J Infect Dis. 1982 Nov;146(5):657–664. doi: 10.1093/infdis/146.5.657. [DOI] [PubMed] [Google Scholar]
- Hartsough G. R., Burger D. Encephalopathy of mink. I. Epizootiologic and clinical observations. J Infect Dis. 1965 Oct;115(4):387–392. doi: 10.1093/infdis/115.4.387. [DOI] [PubMed] [Google Scholar]
- Hope J., Reekie L. J., Hunter N., Multhaup G., Beyreuther K., White H., Scott A. C., Stack M. J., Dawson M., Wells G. A. Fibrils from brains of cows with new cattle disease contain scrapie-associated protein. Nature. 1988 Nov 24;336(6197):390–392. doi: 10.1038/336390a0. [DOI] [PubMed] [Google Scholar]
- Hsiao K., Baker H. F., Crow T. J., Poulter M., Owen F., Terwilliger J. D., Westaway D., Ott J., Prusiner S. B. Linkage of a prion protein missense variant to Gerstmann-Sträussler syndrome. Nature. 1989 Mar 23;338(6213):342–345. doi: 10.1038/338342a0. [DOI] [PubMed] [Google Scholar]
- Kimberlin R. H., Walker C. A. Pathogenesis of mouse scrapie: dynamics of agent replication in spleen, spinal cord and brain after infection by different routes. J Comp Pathol. 1979 Oct;89(4):551–562. doi: 10.1016/0021-9975(79)90046-x. [DOI] [PubMed] [Google Scholar]
- Kimberlin R. H., Walker C. A. Pathogenesis of mouse scrapie: patterns of agent replication in different parts of the CNS following intraperitoneal infection. J R Soc Med. 1982 Aug;75(8):618–624. doi: 10.1177/014107688207500809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin R. H., Walker C. A. Pathogenesis of scrapie (strain 263K) in hamsters infected intracerebrally, intraperitoneally or intraocularly. J Gen Virol. 1986 Feb;67(Pt 2):255–263. doi: 10.1099/0022-1317-67-2-255. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Mohri S., Tateishi J. Organ distribution of proteinase-resistant prion protein in humans and mice with Creutzfeldt-Jakob disease. J Gen Virol. 1989 Dec;70(Pt 12):3371–3379. doi: 10.1099/0022-1317-70-12-3371. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Muramoto T., Hilbich C., Beyreuther K., Tateishi J. N-terminal sequence of prion protein is also integrated into kuru plaques in patients with Gerstmann-Sträussler syndrome. Brain Res. 1991 Apr 5;545(1-2):319–321. doi: 10.1016/0006-8993(91)91306-l. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Muramoto T., Mohri S., Doh-Ura K., Tateishi J. Abnormal isoform of prion protein accumulates in follicular dendritic cells in mice with Creutzfeldt-Jakob disease. J Virol. 1991 Nov;65(11):6292–6295. doi: 10.1128/jvi.65.11.6292-6295.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T., Shin R. W., Doh-ura K., Tomokane N., Miyazono M., Muramoto T., Tateishi J. Abnormal isoform of prion proteins accumulates in the synaptic structures of the central nervous system in patients with Creutzfeldt-Jakob disease. Am J Pathol. 1992 Jun;140(6):1285–1294. [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T., Tateishi J. Immunohistochemical confirmation of Creutzfeldt-Jakob disease with a long clinical course with amyloid plaque core antibodies. Am J Pathol. 1988 Jun;131(3):435–443. [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T., Tateishi J., Sawa H., Doh-Ura K. Positive transmission of Creutzfeldt-Jakob disease verified by murine kuru plaques. Lab Invest. 1989 Apr;60(4):507–512. [PubMed] [Google Scholar]
- Kitamoto T., Yamaguchi K., Doh-ura K., Tateishi J. A prion protein missense variant is integrated in kuru plaque cores in patients with Gerstmann-Sträussler syndrome. Neurology. 1991 Feb;41(2 ):306–310. doi: 10.1212/wnl.41.2_part_1.306. [DOI] [PubMed] [Google Scholar]
- Kretzschmar H. A., Stowring L. E., Westaway D., Stubblebine W. H., Prusiner S. B., Dearmond S. J. Molecular cloning of a human prion protein cDNA. DNA. 1986 Aug;5(4):315–324. doi: 10.1089/dna.1986.5.315. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Gajdusek D. C., Gibbs C. J., Jr Creutzfeldt-Jakob disease virus isolations from the Gerstmann-Sträussler syndrome with an analysis of the various forms of amyloid plaque deposition in the virus-induced spongiform encephalopathies. Brain. 1981 Sep;104(3):559–588. doi: 10.1093/brain/104.3.559. [DOI] [PubMed] [Google Scholar]
- Meyer R. K., McKinley M. P., Bowman K. A., Braunfeld M. B., Barry R. A., Prusiner S. B. Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2310–2314. doi: 10.1073/pnas.83.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto T., Kitamoto T., Tateishi J., Goto I. Successful transmission of Creutzfeldt-Jakob disease from human to mouse verified by prion protein accumulation in mouse brains. Brain Res. 1992 Dec 25;599(2):309–316. doi: 10.1016/0006-8993(92)90406-y. [DOI] [PubMed] [Google Scholar]
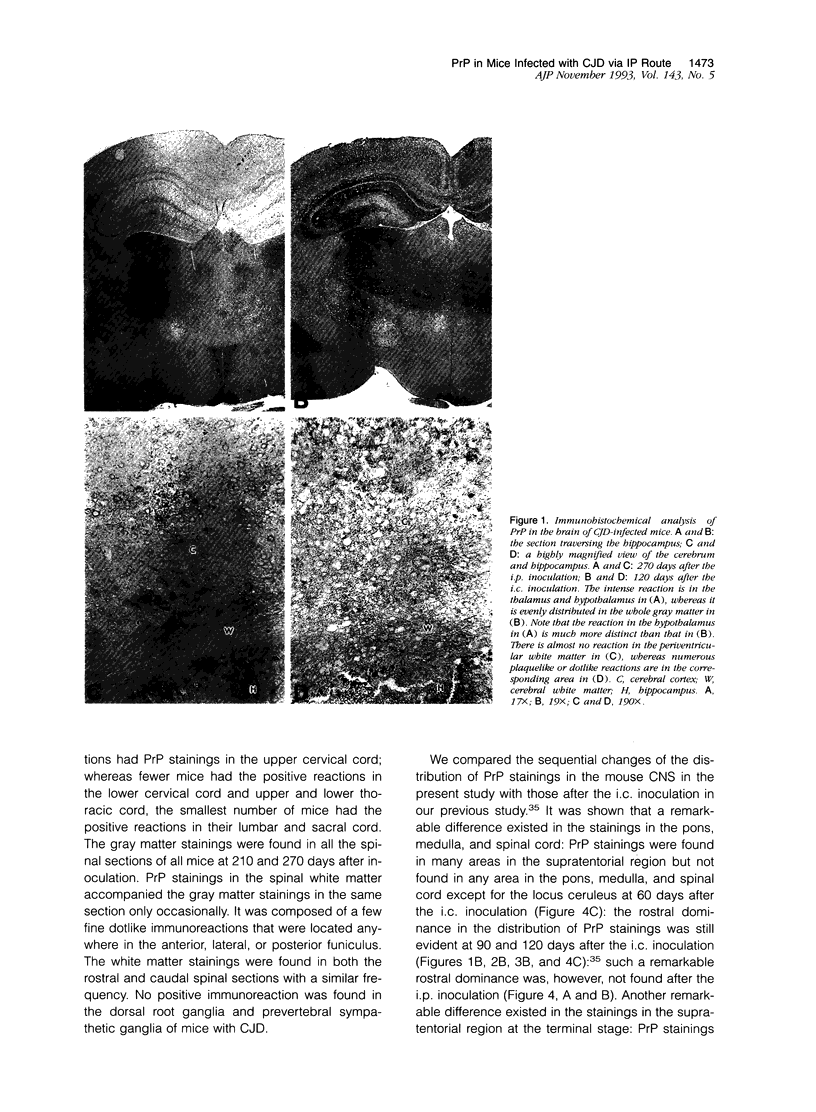
- Muramoto T., Kitamoto T., Tateishi J., Goto I. The sequential development of abnormal prion protein accumulation in mice with Creutzfeldt-Jakob disease. Am J Pathol. 1992 Jun;140(6):1411–1420. [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
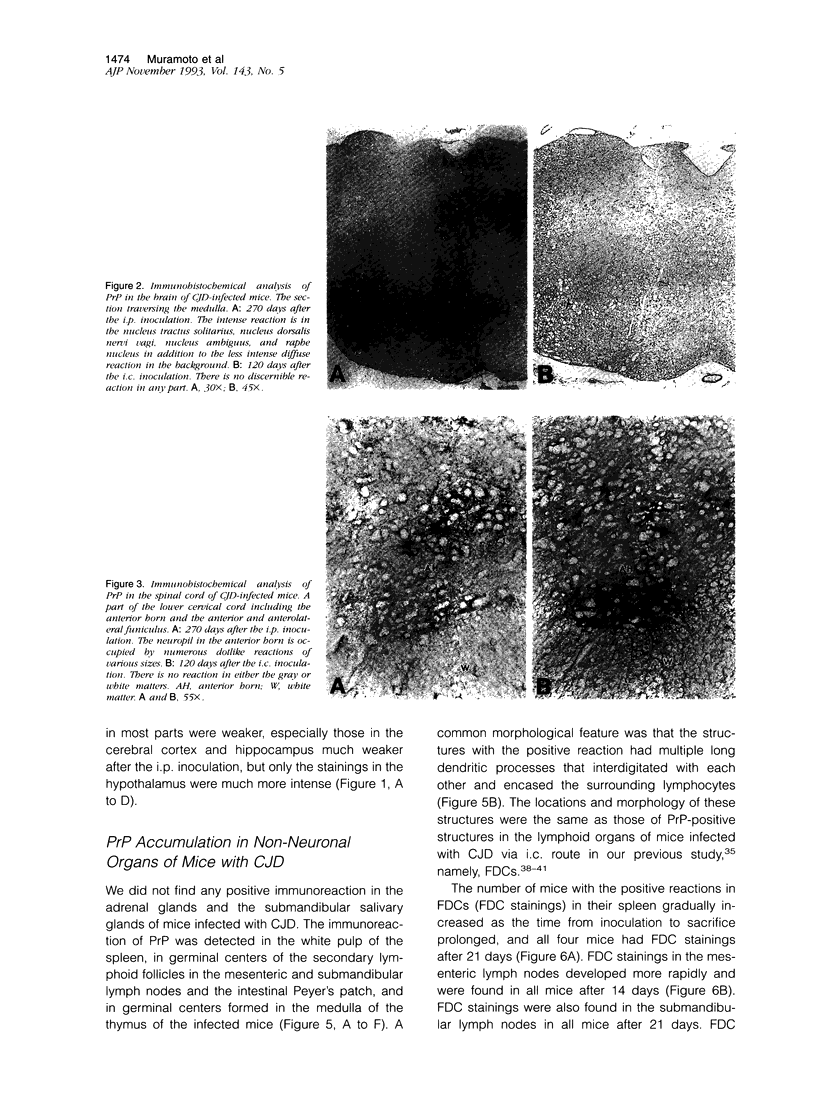
- Outram G. W., Fraser H., Wilson D. T. Scrapie in mice. Some effects on the brain lesion profile of ME7 agent due to genotype of donor, route of injection and genotype of recipient. J Comp Pathol. 1973 Jan;83(1):19–28. doi: 10.1016/0021-9975(73)90023-6. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Bolton D. C., Groth D. F., Bowman K. A., Cochran S. P., McKinley M. P. Further purification and characterization of scrapie prions. Biochemistry. 1982 Dec 21;21(26):6942–6950. doi: 10.1021/bi00269a050. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Bolton D. C., Kent S. B., Hood L. E. Purification and structural studies of a major scrapie prion protein. Cell. 1984 Aug;38(1):127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Schnizlein C. T., Szakal A. K., Tew J. G. Follicular dendritic cells in the regulation and maintenance of immune responses. Immunobiology. 1984 Dec;168(3-5):391–402. doi: 10.1016/S0171-2985(84)80125-4. [DOI] [PubMed] [Google Scholar]
- Tateishi J., Doi H., Sato Y., Suetsugu M., Ishii K., Kuroiwa Y. Experimental transmission of human subacute spongiform encephalopathy to small rodents. III. Further transmission from three patients and distribution patterns of lesions in mice. Acta Neuropathol. 1981;53(2):161–163. doi: 10.1007/BF00689997. [DOI] [PubMed] [Google Scholar]
- Tateishi J., Ohta M., Koga M., Sato Y., Kuroiwa Y. Transmission of chronic spongiform encephalopathy with kuru plaques from humans to small rodents. Ann Neurol. 1979 Jun;5(6):581–584. doi: 10.1002/ana.410050616. [DOI] [PubMed] [Google Scholar]
- Tateishi J., Sato Y., Koga M., Doi H., Ohta M. Experimental transmission of human subacute spongiform encephalopathy to small rodents. I. Clinical and histological observations. Acta Neuropathol. 1980;51(2):127–134. doi: 10.1007/BF00690454. [DOI] [PubMed] [Google Scholar]
- Tew J. G., Phipps R. P., Mandel T. E. The maintenance and regulation of the humoral immune response: persisting antigen and the role of follicular antigen-binding dendritic cells as accessory cells. Immunol Rev. 1980;53:175–201. doi: 10.1111/j.1600-065x.1980.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Witmer M. D., Steinman R. M. The phenotype of dendritic cells and macrophages. Fed Proc. 1983 Nov;42(14):3114–3118. [PubMed] [Google Scholar]
- Westaway D., Goodman P. A., Mirenda C. A., McKinley M. P., Carlson G. A., Prusiner S. B. Distinct prion proteins in short and long scrapie incubation period mice. Cell. 1987 Nov 20;51(4):651–662. doi: 10.1016/0092-8674(87)90134-6. [DOI] [PubMed] [Google Scholar]
- Williams E. S., Young S. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis. 1980 Jan;16(1):89–98. doi: 10.7589/0090-3558-16.1.89. [DOI] [PubMed] [Google Scholar]