Abstract
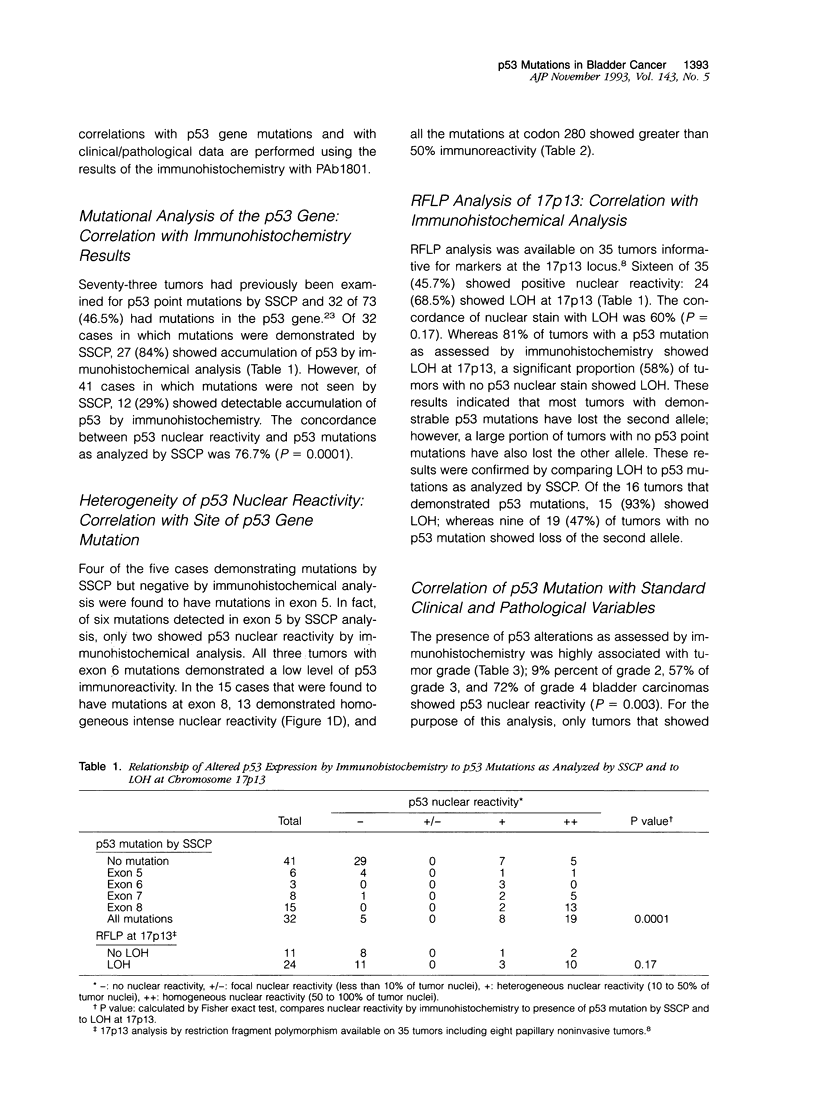
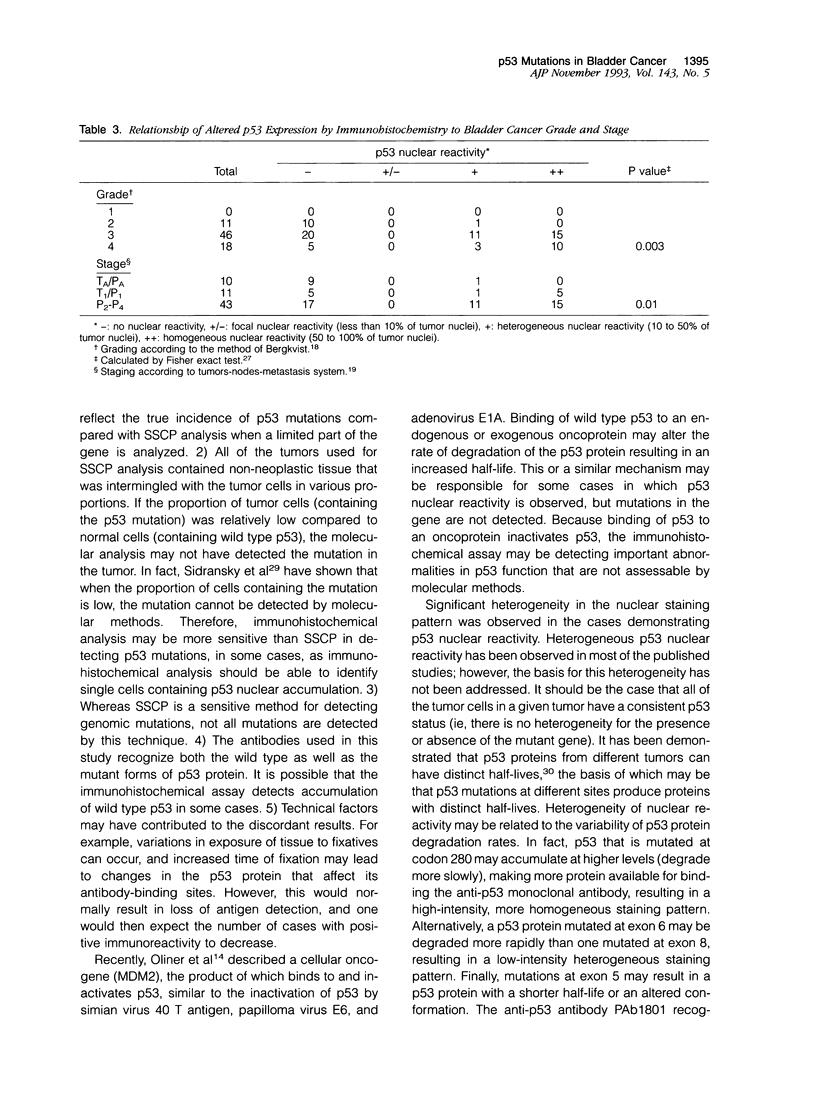
Seventy-three transitional cell carcinomas of the bladder were analyzed by immunohistochemistry for p53 nuclear accumulation, and the results were compared to mutations detected in the p53 gene by single strand conformational polymorphism analysis (SSCP) and DNA sequence analysis. Immunohistochemical studies were performed on formalin-fixed, paraffin-embedded tissue sections. A highly significant association between the presence of p53 mutations and p53 nuclear reactivity as detected by immunohistochemistry was found (P = 0.0001). Of 32 tumors that demonstrated p53 mutations by SSCP, 27 (84%) showed p53 nuclear reactivity. Of the five cases that did not demonstrate p53 nuclear reactivity, four had mutations in exon 5. However, of 41 tumors with no evidence of p53 mutation by molecular analysis, 12 (29%) showed p53 immunoreactivity. This indicates that immunohistochemical methods may be more sensitive than SSCP in detecting p53 mutations or that discordant cases represent tumors with accumulation of wild type p53 protein, without mutations at the p53 locus. Of the 15 tumors that were found to have mutations at exon 8, 13 demonstrated high-intensity homogeneous p53 nuclear reactivity by immunohistochemistry, and all mutations located at codon 280 demonstrated high-intensity homogeneous immunoreactivity. However, three of three tumors with exon 6 mutations demonstrated low-level p53 immunoreactivity, and four of six tumors with mutations in exon 5 showed no detectable p53 nuclear reactivity. This indicates that the heterogeneity of immunoreactivity observed when analyzing p53 nuclear accumulation may be related to the site of the p53 gene mutation. Information on tumor grade, stage, lymph node status, disease-free interval, and overall survival were available in 54 patients who had undergone cystectomy. A significant association was observed between p53 alterations (detected by immunohistochemistry and SSCP) and histological tumor grade (P = 0.003) and stage (P = 0.01). We conclude that the immunohistochemical detection of p53 nuclear accumulation in formalin-fixed, paraffin-embedded tissue is highly associated with mutations in the p53 gene; this association has now been demonstrated in a large number of tumors. The heterogeneity of p53 nuclear reactivity seems to be related to the site of mutation in the p53 gene. A small proportion of tumors with a p53 gene mutation do not demonstrate immunohistochemically detectable p53 nuclear accumulation. Furthermore, a small but substantial proportion of tumors demonstrate p53 nuclear reactivity but do not show detectable mutations in the p53 gene by SSCP. Finally, both grade and stage of bladder cancer are related to p53 alterations, detected by immunohistochemistry or molecular methods.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergkvist A., Ljungqvist A., Moberger G. Classification of bladder tumours based on the cellular pattern. Preliminary report of a clinical-pathological study of 300 cases with a minimum follow-up of eight years. Acta Chir Scand. 1965 Oct;130(4):371–378. [PubMed] [Google Scholar]
- Buchman V. L., Chumakov P. M., Ninkina N. N., Samarina O. P., Georgiev G. P. A variation in the structure of the protein-coding region of the human p53 gene. Gene. 1988 Oct 30;70(2):245–252. doi: 10.1016/0378-1119(88)90196-5. [DOI] [PubMed] [Google Scholar]
- Cairns P., Shaw M. E., Knowles M. A. Preliminary mapping of the deleted region of chromosome 9 in bladder cancer. Cancer Res. 1993 Mar 15;53(6):1230–1232. [PubMed] [Google Scholar]
- Cote R. J., Jhanwar S. C., Novick S., Pellicer A. Genetic alterations of the p53 gene are a feature of malignant mesotheliomas. Cancer Res. 1991 Oct 1;51(19):5410–5416. [PubMed] [Google Scholar]
- Davidoff A. M., Humphrey P. A., Iglehart J. D., Marks J. R. Genetic basis for p53 overexpression in human breast cancer. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5006–5010. doi: 10.1073/pnas.88.11.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliyahu D., Raz A., Gruss P., Givol D., Oren M. Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature. 1984 Dec 13;312(5995):646–649. doi: 10.1038/312646a0. [DOI] [PubMed] [Google Scholar]
- Fields S., Jang S. K. Presence of a potent transcription activating sequence in the p53 protein. Science. 1990 Aug 31;249(4972):1046–1049. doi: 10.1126/science.2144363. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K., Yamada Y., Okajima E., Kakizoe T., Sasaki H., Sugimura T., Terada M. Frequent association of p53 gene mutation in invasive bladder cancer. Cancer Res. 1992 Mar 15;52(6):1393–1398. [PubMed] [Google Scholar]
- Kikuchi-Yanoshita R., Konishi M., Ito S., Seki M., Tanaka K., Maeda Y., Iino H., Fukayama M., Koike M., Mori T. Genetic changes of both p53 alleles associated with the conversion from colorectal adenoma to early carcinoma in familial adenomatous polyposis and non-familial adenomatous polyposis patients. Cancer Res. 1992 Jul 15;52(14):3965–3971. [PubMed] [Google Scholar]
- Knudson A. G., Jr Hereditary cancer, oncogenes, and antioncogenes. Cancer Res. 1985 Apr;45(4):1437–1443. [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Logothetis C. J., Xu H. J., Ro J. Y., Hu S. X., Sahin A., Ordonez N., Benedict W. F. Altered expression of retinoblastoma protein and known prognostic variables in locally advanced bladder cancer. J Natl Cancer Inst. 1992 Aug 19;84(16):1256–1261. doi: 10.1093/jnci/84.16.1256. [DOI] [PubMed] [Google Scholar]
- Mazars R., Spinardi L., BenCheikh M., Simony-Lafontaine J., Jeanteur P., Theillet C. p53 mutations occur in aggressive breast cancer. Cancer Res. 1992 Jul 15;52(14):3918–3923. [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Oliner J. D., Kinzler K. W., Meltzer P. S., George D. L., Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992 Jul 2;358(6381):80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- Olumi A. F., Tsai Y. C., Nichols P. W., Skinner D. G., Cain D. R., Bender L. I., Jones P. A. Allelic loss of chromosome 17p distinguishes high grade from low grade transitional cell carcinomas of the bladder. Cancer Res. 1990 Nov 1;50(21):7081–7083. [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Presti J. C., Jr, Reuter V. E., Galan T., Fair W. R., Cordon-Cardo C. Molecular genetic alterations in superficial and locally advanced human bladder cancer. Cancer Res. 1991 Oct 1;51(19):5405–5409. [PubMed] [Google Scholar]
- Sidransky D., Mikkelsen T., Schwechheimer K., Rosenblum M. L., Cavanee W., Vogelstein B. Clonal expansion of p53 mutant cells is associated with brain tumour progression. Nature. 1992 Feb 27;355(6363):846–847. doi: 10.1038/355846a0. [DOI] [PubMed] [Google Scholar]
- Sidransky D., Von Eschenbach A., Tsai Y. C., Jones P., Summerhayes I., Marshall F., Paul M., Green P., Hamilton S. R., Frost P. Identification of p53 gene mutations in bladder cancers and urine samples. Science. 1991 May 3;252(5006):706–709. doi: 10.1126/science.2024123. [DOI] [PubMed] [Google Scholar]
- Spruck C. H., 3rd, Rideout W. M., 3rd, Olumi A. F., Ohneseit P. F., Yang A. S., Tsai Y. C., Nichols P. W., Horn T., Hermann G. G., Steven K. Distinct pattern of p53 mutations in bladder cancer: relationship to tobacco usage. Cancer Res. 1993 Mar 1;53(5):1162–1166. [PubMed] [Google Scholar]
- Tsai Y. C., Nichols P. W., Hiti A. L., Williams Z., Skinner D. G., Jones P. A. Allelic losses of chromosomes 9, 11, and 17 in human bladder cancer. Cancer Res. 1990 Jan 1;50(1):44–47. [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Yokota J., Wada M., Shimosato Y., Terada M., Sugimura T. Loss of heterozygosity on chromosomes 3, 13, and 17 in small-cell carcinoma and on chromosome 3 in adenocarcinoma of the lung. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9252–9256. doi: 10.1073/pnas.84.24.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]




