Abstract
In transgenic mice bearing the Simian Virus 40 large T antigen under the control of the human antithrombin III regulatory sequences, a stepwise progression toward hepatocellular carcinoma is observed. We have used two monoclonal antibodies (A6 and G7) developed against a surface antigen expressed in oval cells from dipin-treated mice, to analyze the emergence of such preneoplastic populations in the livers of antithrombin III Simian Virus 40 T transgenic mice. We show that a unique population of small heterogeneous epithelial cells, which probably corresponds to oval and/or transitional cells according to their morphological features, consistently appears at approximately the 10th week after birth and proliferates thereafter. This oval cell-like population stained positively for A6 and G7 monoclonal antibodies. Furthermore, different subpopulations usually recognized as possible precursors of carcinoma cells including hyperplastic foci and neoplastic nodules as well as carcinoma cells, were also positive for A6 but not G7 monoclonal antibodies. Stimulation of cell proliferation by partial hepatectomy performed at the time of emergence of the oval-like cells resulted in a rapid increase in the number of oval/transitional A6-positive cells. Our findings support the view that a common mechanism may be involved in the development of carcinomas that are induced by chemical carcinogens and in transgenic mice expressing a potent oncogene under the control of a hepatic specific promoter. In addition, our findings demonstrate a specific precursor-product relationship between the appearance of the oval/transitional cells and the development of neoplastic hepatocytes in this transgenic model.
Full text
PDF



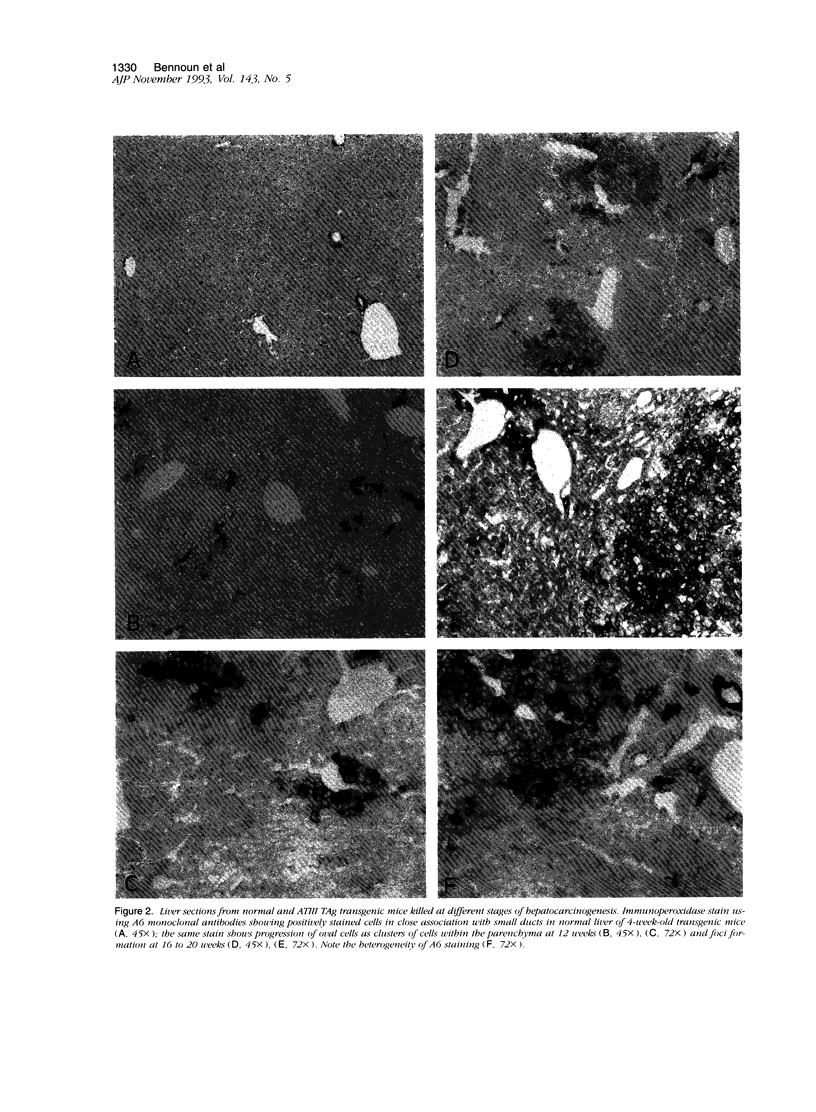






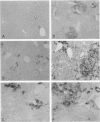
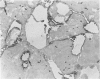
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun L., Mikumo R., Fausto N. Production of hepatocellular carcinoma by oval cells: cell cycle expression of c-myc and p53 at different stages of oval cell transformation. Cancer Res. 1989 Mar 15;49(6):1554–1561. [PubMed] [Google Scholar]
- D'Souza S. E., Mercer J. F. Antithrombin III mRNA in adult rat liver and kidney and in rat liver during development. Biochem Biophys Res Commun. 1987 Jan 30;142(2):417–421. doi: 10.1016/0006-291x(87)90290-7. [DOI] [PubMed] [Google Scholar]
- Dubois N., Bennoun M., Allemand I., Molina T., Grimber G., Daudet-Monsac M., Abelanet R., Briand P. Time-course development of differentiated hepatocarcinoma and lung metastasis in transgenic mice. J Hepatol. 1991 Sep;13(2):227–239. doi: 10.1016/0168-8278(91)90819-w. [DOI] [PubMed] [Google Scholar]
- Dunsford H. A., Karnasuta C., Hunt J. M., Sell S. Different lineages of chemically induced hepatocellular carcinoma in rats defined by monoclonal antibodies. Cancer Res. 1989 Sep 1;49(17):4894–4900. [PubMed] [Google Scholar]
- Dunsford H. A., Sell S., Chisari F. V. Hepatocarcinogenesis due to chronic liver cell injury in hepatitis B virus transgenic mice. Cancer Res. 1990 Jun 1;50(11):3400–3407. [PubMed] [Google Scholar]
- Dunsford H. A., Sell S. Production of monoclonal antibodies to preneoplastic liver cell populations induced by chemical carcinogens in rats and to transplantable Morris hepatomas. Cancer Res. 1989 Sep 1;49(17):4887–4893. [PubMed] [Google Scholar]
- Dyer K. R., Messing A. Metal-inducible pathology in the liver, pancreas, and kidney of transgenic mice expressing SV40 early region genes. Am J Pathol. 1989 Aug;135(2):401–410. [PMC free article] [PubMed] [Google Scholar]
- Engelhardt N. V., Baranov V. N., Lazareva M. N., Goussev A. I. Ultrastructural localisation of alpha-fetoprotin (AFP) in regenerating mouse liver poisoned with CCL4. 1. Reexpression of AFP in differentiated hepatocytes. Histochemistry. 1984;80(4):401–407. doi: 10.1007/BF00495425. [DOI] [PubMed] [Google Scholar]
- Engelhardt N. V., Factor V. M., Yasova A. K., Poltoranina V. S., Baranov V. N., Lasareva M. N. Common antigens of mouse oval and biliary epithelial cells. Expression on newly formed hepatocytes. Differentiation. 1990 Oct;45(1):29–37. doi: 10.1111/j.1432-0436.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Marsden E., Thorgeirsson S. S. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987 Nov;8(11):1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- Farber E. The multistep nature of cancer development. Cancer Res. 1984 Oct;44(10):4217–4223. [PubMed] [Google Scholar]
- Faris R. A., Monfils B. A., Dunsford H. A., Hixson D. C. Antigenic relationship between oval cells and a subpopulation of hepatic foci, nodules, and carcinomas induced by the "resistant hepatocyte" model system. Cancer Res. 1991 Feb 15;51(4):1308–1317. [PubMed] [Google Scholar]
- Fausto N. Hepatocyte differentiation and liver progenitor cells. Curr Opin Cell Biol. 1990 Dec;2(6):1036–1042. doi: 10.1016/0955-0674(90)90153-6. [DOI] [PubMed] [Google Scholar]
- GRISHAM J. W., PORTA E. A. ORIGIN AND FATE OF PROLIFERATED HEPATIC DUCTAL CELLS IN THE RAT: ELECTRON MICROSCOPIC AND AUTORADIOGRAPHIC STUDIES. Exp Mol Pathol. 1964 Jun;86:242–261. doi: 10.1016/0014-4800(64)90057-7. [DOI] [PubMed] [Google Scholar]
- Goldfarb S., Pugh T. D., Cripps D. J. Increased alkaline phosphatase activity--a positive histochemical marker for griseofulvin-induced mouse hepatocellular nodules. J Natl Cancer Inst. 1980 Jun;64(6):1427–1433. doi: 10.1093/jnci/64.6.1427. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985 May 9;315(6015):115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- Held W. A., Mullins J. J., Kuhn N. J., Gallagher J. F., Gu G. D., Gross K. W. T antigen expression and tumorigenesis in transgenic mice containing a mouse major urinary protein/SV40 T antigen hybrid gene. EMBO J. 1989 Jan;8(1):183–191. doi: 10.1002/j.1460-2075.1989.tb03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixson D. C., Allison J. P. Monoclonal antibodies recognizing oval cells induced in the liver of rats by N-2-fluorenylacetamide or ethionine in a choline-deficient diet. Cancer Res. 1985 Aug;45(8):3750–3760. [PubMed] [Google Scholar]
- Inaoka Y. Significance of the so-called oval cell proliferation during azo-dye hepatocarcinogenesis. Gan. 1967 Aug;58(4):355–366. [PubMed] [Google Scholar]
- Lemire J. M., Shiojiri N., Fausto N. Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am J Pathol. 1991 Sep;139(3):535–552. [PMC free article] [PubMed] [Google Scholar]
- Lipsky M. M., Hinton D. E., Klaunig J. E., Trump B. F. Biology of hepatocellular neoplasia in the mouse. I. Histogenesis of safrole-induced hepatocellular carcinoma. J Natl Cancer Inst. 1981 Aug;67(2):365–376. [PubMed] [Google Scholar]
- Marceau N., Blouin M. J., Germain L., Noel M. Role of different epithelial cell types in liver ontogenesis, regeneration and neoplasia. In Vitro Cell Dev Biol. 1989 Apr;25(4):336–341. doi: 10.1007/BF02624596. [DOI] [PubMed] [Google Scholar]
- Marceau N. Cell lineages and differentiation programs in epidermal, urothelial and hepatic tissues and their neoplasms. Lab Invest. 1990 Jul;63(1):4–20. [PubMed] [Google Scholar]
- Marceau N., Germain L., Goyette R., Noël M., Gourdeau H. Cell of origin of distinct cultured rat liver epithelial cells, as typed by cytokeratin and surface component selective expression. Biochem Cell Biol. 1986 Aug;64(8):788–802. doi: 10.1139/o86-107. [DOI] [PubMed] [Google Scholar]
- Maunoury R., Robine S., Pringault E., Huet C., Guénet J. L., Gaillard J. A., Louvard D. Villin expression in the visceral endoderm and in the gut anlage during early mouse embryogenesis. EMBO J. 1988 Nov;7(11):3321–3329. doi: 10.1002/j.1460-2075.1988.tb03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K., Minase T., Onhoe T. Demonstration of glucose 6-phosphatase activity in the oval cells of rat liver and the significance of the oval cells in azo dye carcinogenesis. Cancer Res. 1974 Dec;34(12):3379–3386. [PubMed] [Google Scholar]
- Petropoulos C. J., Yaswen P., Panzica M., Fausto N. Cell lineages in liver carcinogenesis: possible clues from studies of the distribution of alpha-fetoprotein RNA sequences in cell populations isolated from normal, regenerating, and preneoplastic rat livers. Cancer Res. 1985 Nov;45(11 Pt 2):5762–5768. [PubMed] [Google Scholar]
- Sandgren E. P., Quaife C. J., Pinkert C. A., Palmiter R. D., Brinster R. L. Oncogene-induced liver neoplasia in transgenic mice. Oncogene. 1989 Jun;4(6):715–724. [PubMed] [Google Scholar]
- Schirmacher P., Held W. A., Yang D., Biempica L., Rogler C. E. Selective amplification of periportal transitional cells precedes formation of hepatocellular carcinoma in SV40 large tag transgenic mice. Am J Pathol. 1991 Jul;139(1):231–241. [PMC free article] [PubMed] [Google Scholar]
- Sell S. Distribution of alpha-fetoprotein- and albumin-containing cells in the livers of Fischer rats fed four cycles of N-2-fluorenylacetamide. Cancer Res. 1978 Sep;38(9):3107–3113. [PubMed] [Google Scholar]
- Sell S., Dunsford H. A. Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989 Jun;134(6):1347–1363. [PMC free article] [PubMed] [Google Scholar]
- Sell S. Is there a liver stem cell? Cancer Res. 1990 Jul 1;50(13):3811–3815. [PubMed] [Google Scholar]
- Sepulveda A. R., Finegold M. J., Smith B., Slagle B. L., DeMayo J. L., Shen R. F., Woo S. L., Butel J. S. Development of a transgenic mouse system for the analysis of stages in liver carcinogenesis using tissue-specific expression of SV40 large T-antigen controlled by regulatory elements of the human alpha-1-antitrypsin gene. Cancer Res. 1989 Nov 1;49(21):6108–6117. [PubMed] [Google Scholar]
- Solt D. B., Medline A., Farber E. Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am J Pathol. 1977 Sep;88(3):595–618. [PMC free article] [PubMed] [Google Scholar]
- Steinberg P., Hacker H. J., Dienes H. P., Oesch F., Bannasch P. Enzyme histochemical and immunohistochemical characterization of oval and parenchymal cells proliferating in livers of rats fed a choline-deficient/DL-ethionine-supplemented diet. Carcinogenesis. 1991 Feb;12(2):225–231. doi: 10.1093/carcin/12.2.225. [DOI] [PubMed] [Google Scholar]
- Tsao M. S., Grisham J. W. Hepatocarcinomas, cholangiocarcinomas, and hepatoblastomas produced by chemically transformed cultured rat liver epithelial cells. A light- and electron-microscopic analysis. Am J Pathol. 1987 Apr;127(1):168–181. [PMC free article] [PubMed] [Google Scholar]
- Weber A., Le Provost E., Boisnard-Rissel M., Berges J., Schapira F., Guillouzo A. Localization of fetal aldolases during early stages of azo-dye hepatocarcinogenesis in rat. Biochem Biophys Res Commun. 1980 Jan 29;92(2):591–597. doi: 10.1016/0006-291x(80)90374-5. [DOI] [PubMed] [Google Scholar]