Abstract
Tenascin is a major extracellular matrix glycoprotein that can interfere with the action of fibronectin by inhibiting cell adhesion and spreading. Although tenascin is able to exert important immunomodulatory activities on T and B cells and macrophages, little is known about its distribution in different lymphohemopoietic tissues. In this study we have analyzed tenascin immunoreactivity on cryostat and paraffin sections of normal and pathological lymphoid tissues using two different monoclonal antibodies. We demonstrated strong tenascin expression in all peripheral lymphoid tissues, whereas it was barely detectable in the thymus and in bone marrow. In reactive lymph nodes, tenascin was mainly found in T-dependent zones, forming a variably close-woven reticular network corresponding to fibroblastic reticulum cells and blood vessels basal laminae, showing a partial co-localization with fibronectin. In B-dependent zones, tenascin was restricted to blood vessels. Using double-marker analysis, we performed a thorough study comparing tenascin expression in different compartments of lymphoid microenvironments. Tenascin network appeared much thicker in chronically stimulated tissues, where CD4+ lymphocytes with "memory" phenotype (CD45RO+/CD45RA-) were predominant, and at sites of ongoing inflammation. In particular, a striking increase of tenascin was observed in sarcoid lymph node, as well as in myasthenic hyperplastic thymuses. In addition, tenascin can be abnormally synthesized in tissue involved by various types of lymphomas, including Hodgkin's disease and hairy cell leukemia.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anbazhagan R., Sakakura T., Gusterson B. A. The distribution of immuno-reactive tenascin in the epithelial-mesenchymal junctional areas of benign and malignant squamous epithelia. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(1):59–63. doi: 10.1007/BF02899388. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
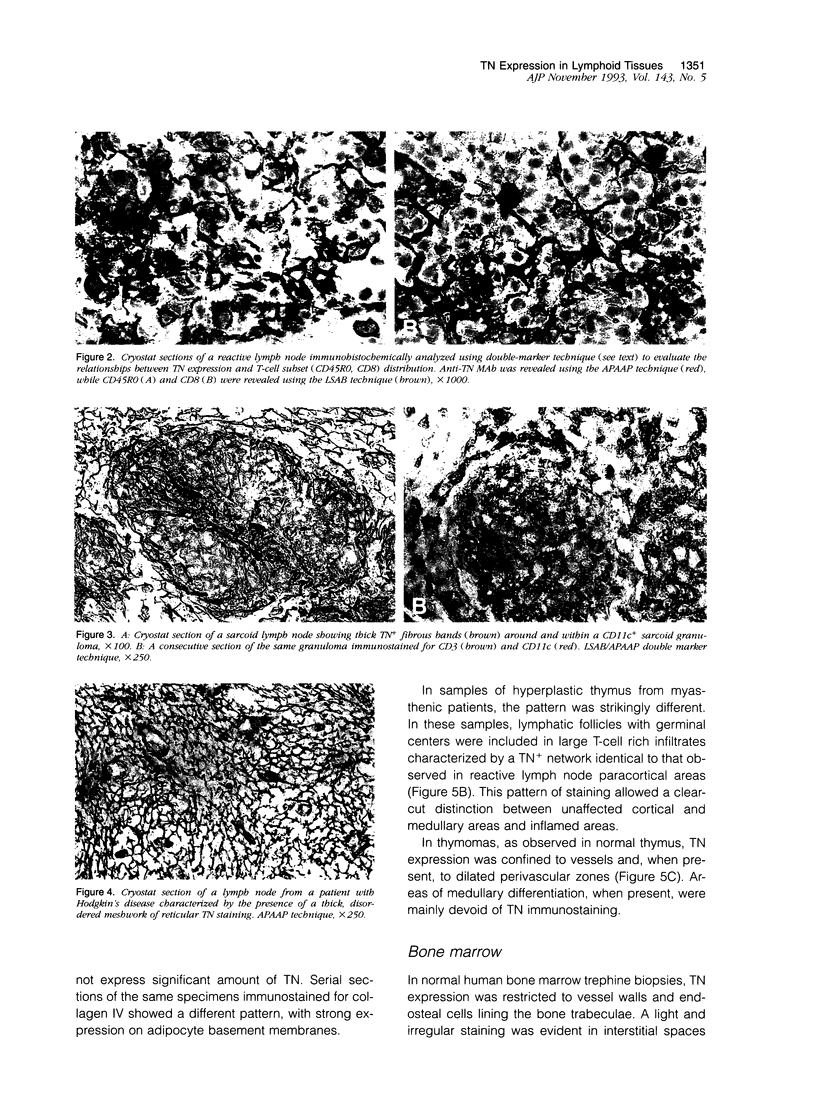
- Bofill M., Janossy G., Willcox N., Chilosi M., Trejdosiewicz L. K., Newsom-Davis J. Microenvironments in the normal thymus and the thymus in myasthenia gravis. Am J Pathol. 1985 Jun;119(3):462–473. [PMC free article] [PubMed] [Google Scholar]
- Chilosi M., Iannucci A., Fiore-Donati L., Tridente G., Pampanin M., Pizzolo G., Ritter M., Bofill M., Janossy G. Myasthenia gravis: immunohistological heterogeneity in microenvironmental organization of hyperplastic and neoplastic thymuses suggesting different mechanisms of tolerance breakdown. J Neuroimmunol. 1986 May;11(3):191–204. doi: 10.1016/0165-5728(86)90003-2. [DOI] [PubMed] [Google Scholar]
- Chilosi M., Menestrina F., Capelli P., Montagna L., Lestani M., Pizzolo G., Cipriani A., Agostini C., Trentin L., Zambello R. Immunohistochemical analysis of sarcoid granulomas. Evaluation of Ki67+ and interleukin-1+ cells. Am J Pathol. 1988 May;131(2):191–198. [PMC free article] [PubMed] [Google Scholar]
- Chilosi M., Mombello A., Montagna L., Benedetti A., Lestani M., Semenzato G., Menestrina F. Multimarker immunohistochemical staining of calgranulins, chloroacetate esterase, and S100 for simultaneous demonstration of inflammatory cells on paraffin sections. J Histochem Cytochem. 1990 Nov;38(11):1669–1675. doi: 10.1177/38.11.2212622. [DOI] [PubMed] [Google Scholar]
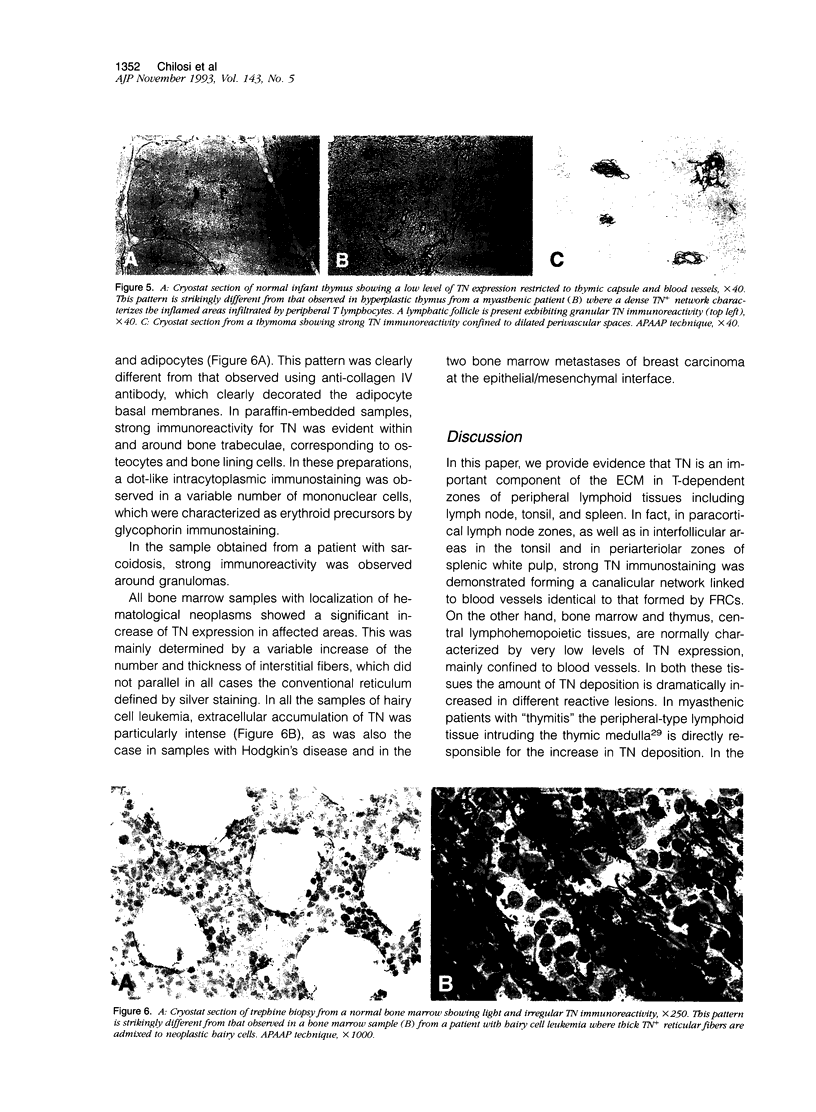
- Chilosi M., Pizzolo G., Fiore-Donati L., Bofill M., Janossy G. Routine immunofluorescent and histochemical analysis of bone marrow involvement of lymphoma/leukaemia: the use of cryostat sections. Br J Cancer. 1983 Dec;48(6):763–775. doi: 10.1038/bjc.1983.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Kalla P., Pearson C. A., Beck K., Chiquet M. Tenascin interferes with fibronectin action. Cell. 1988 May 6;53(3):383–390. doi: 10.1016/0092-8674(88)90158-4. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Kalla P., Pearson C. A. Participation of tenascin and transforming growth factor-beta in reciprocal epithelial-mesenchymal interactions of MCF7 cells and fibroblasts. Cancer Res. 1989 Aug 1;49(15):4322–4325. [PubMed] [Google Scholar]
- Chiquet-Ehrismann R. What distinguishes tenascin from fibronectin? FASEB J. 1990 Jun;4(9):2598–2604. doi: 10.1096/fasebj.4.9.1693347. [DOI] [PubMed] [Google Scholar]
- Chuong C. M., Chen H. M. Enhanced expression of neural cell adhesion molecules and tenascin (cytotactin) during wound healing. Am J Pathol. 1991 Feb;138(2):427–440. [PMC free article] [PubMed] [Google Scholar]
- Davis L. S., Oppenheimer-Marks N., Bednarczyk J. L., McIntyre B. W., Lipsky P. E. Fibronectin promotes proliferation of naive and memory T cells by signaling through both the VLA-4 and VLA-5 integrin molecules. J Immunol. 1990 Aug 1;145(3):785–793. [PubMed] [Google Scholar]
- Erickson H. P., Bourdon M. A. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Herlyn M., Graeven U., Speicher D., Sela B. A., Bennicelli J. L., Kath R., Guerry D., 4th Characterization of tenascin secreted by human melanoma cells. Cancer Res. 1991 Sep 15;51(18):4853–4858. [PubMed] [Google Scholar]
- Inaguma Y., Kusakabe M., Mackie E. J., Pearson C. A., Chiquet-Ehrismann R., Sakakura T. Epithelial induction of stromal tenascin in the mouse mammary gland: from embryogenesis to carcinogenesis. Dev Biol. 1988 Aug;128(2):245–255. doi: 10.1016/0012-1606(88)90288-6. [DOI] [PubMed] [Google Scholar]
- Janossy G., Bofill M., Trejdosiewicz L. K., Willcox H. N., Chilosi M. Cellular differentiation of lymphoid subpopulations and their microenvironments in the human thymus. Curr Top Pathol. 1986;75:89–125. doi: 10.1007/978-3-642-82480-7_3. [DOI] [PubMed] [Google Scholar]
- Kadin M. E., Agnarsson B. A., Ellingsworth L. R., Newcom S. R. Immunohistochemical evidence of a role for transforming growth factor beta in the pathogenesis of nodular sclerosing Hodgkin's disease. Am J Pathol. 1990 Jun;136(6):1209–1214. [PMC free article] [PubMed] [Google Scholar]
- Kadin M., Butmarc J., Elovic A., Wong D. Eosinophils are the major source of transforming growth factor-beta 1 in nodular sclerosing Hodgkin's disease. Am J Pathol. 1993 Jan;142(1):11–16. [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukoulis G. K., Gould V. E., Bhattacharyya A., Gould J. E., Howeedy A. A., Virtanen I. Tenascin in normal, reactive, hyperplastic, and neoplastic tissues: biologic and pathologic implications. Hum Pathol. 1991 Jul;22(7):636–643. doi: 10.1016/0046-8177(91)90285-w. [DOI] [PubMed] [Google Scholar]
- Larson R. S., Springer T. A. Structure and function of leukocyte integrins. Immunol Rev. 1990 Apr;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Liakka K. A., Autio-Harmainen H. I. Distribution of the extracellular matrix proteins tenascin, fibronectin, and vitronectin in fetal, infant, and adult human spleens. J Histochem Cytochem. 1992 Aug;40(8):1203–1210. doi: 10.1177/40.8.1377736. [DOI] [PubMed] [Google Scholar]
- Lightner V. A., Erickson H. P. Binding of hexabrachion (tenascin) to the extracellular matrix and substratum and its effect on cell adhesion. J Cell Sci. 1990 Feb;95(Pt 2):263–277. doi: 10.1242/jcs.95.2.263. [DOI] [PubMed] [Google Scholar]
- Lightner V. A., Gumkowski F., Bigner D. D., Erickson H. P. Tenascin/hexabrachion in human skin: biochemical identification and localization by light and electron microscopy. J Cell Biol. 1989 Jun;108(6):2483–2493. doi: 10.1083/jcb.108.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque J. P., Hatzfeld A., Hatzfeld J. Mitogenic properties of major extracellular proteins. Immunol Today. 1991 Aug;12(8):258–262. doi: 10.1016/0167-5699(91)90122-A. [DOI] [PubMed] [Google Scholar]
- Mackie E. J., Chiquet-Ehrismann R., Pearson C. A., Inaguma Y., Taya K., Kawarada Y., Sakakura T. Tenascin is a stromal marker for epithelial malignancy in the mammary gland. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4621–4625. doi: 10.1073/pnas.84.13.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali P. G., Nicotra M. R., Bigotti A., Botti C., Castellani P., Risso A. M., Zardi L. Comparative analysis of the expression of the extracellular matrix protein tenascin in normal human fetal, adult and tumor tissues. Int J Cancer. 1991 Apr 1;47(6):811–816. doi: 10.1002/ijc.2910470603. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Zardi L. Tenascin: a hexameric adhesive glycoprotein. Int J Cancer Suppl. 1989;4:66–68. doi: 10.1002/ijc.2910440718. [DOI] [PubMed] [Google Scholar]
- Oyama F., Hirohashi S., Shimosato Y., Titani K., Sekiguchi K. Qualitative and quantitative changes of human tenascin expression in transformed lung fibroblast and lung tumor tissues: comparison with fibronectin. Cancer Res. 1991 Sep 15;51(18):4876–4881. [PubMed] [Google Scholar]
- Pizzolo G., Vinante F., Nadali G., Ricetti M. M., Morosato L., Marrocchella R., Vincenzi C., Semenzato G., Chilosi M. ICAM-1 tissue overexpression associated with increased serum levels of its soluble form in Hodgkin's disease. Br J Haematol. 1993 May;84(1):161–162. doi: 10.1111/j.1365-2141.1993.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., McCune B. K., Sporn M. B. TGF-beta: regulation of extracellular matrix. Kidney Int. 1992 Mar;41(3):557–559. doi: 10.1038/ki.1992.81. [DOI] [PubMed] [Google Scholar]
- Rüegg C. R., Chiquet-Ehrismann R., Alkan S. S. Tenascin, an extracellular matrix protein, exerts immunomodulatory activities. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7437–7441. doi: 10.1073/pnas.86.19.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltini C., Kirby M., Trapnell B. C., Tamura N., Crystal R. G. Biased accumulation of T lymphocytes with "memory"-type CD45 leukocyte common antigen gene expression on the epithelial surface of the human lung. J Exp Med. 1990 Apr 1;171(4):1123–1140. doi: 10.1084/jem.171.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Shaw S. Lymphocyte interactions with extracellular matrix. FASEB J. 1991 Jun;5(9):2292–2299. doi: 10.1096/fasebj.5.9.1860621. [DOI] [PubMed] [Google Scholar]
- Speiser D. E., Lees R. K., Hengartner H., Zinkernagel R. M., MacDonald H. R. Positive and negative selection of T cell receptor V beta domains controlled by distinct cell populations in the thymus. J Exp Med. 1989 Dec 1;170(6):2165–2170. doi: 10.1084/jem.170.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Lo D., Gao E. K., Ron Y. T cell selection in the thymus. Immunol Rev. 1988 Jan;101:173–190. doi: 10.1111/j.1600-065x.1988.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Spring J., Beck K., Chiquet-Ehrismann R. Two contrary functions of tenascin: dissection of the active sites by recombinant tenascin fragments. Cell. 1989 Oct 20;59(2):325–334. doi: 10.1016/0092-8674(89)90294-8. [DOI] [PubMed] [Google Scholar]
- Stuart A. E., Warford A. Staining of human splenic sinusoids and demonstration of unusual banded structures by monoclonal antisera. J Clin Pathol. 1983 Oct;36(10):1176–1180. doi: 10.1136/jcp.36.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toccanier-Pelte M. F., Skalli O., Kapanci Y., Gabbiani G. Characterization of stromal cells with myoid features in lymph nodes and spleen in normal and pathologic conditions. Am J Pathol. 1987 Oct;129(1):109–118. [PMC free article] [PubMed] [Google Scholar]
- Vainio S., Jalkanen M., Thesleff I. Syndecan and tenascin expression is induced by epithelial-mesenchymal interactions in embryonic tooth mesenchyme. J Cell Biol. 1989 May;108(5):1945–1953. doi: 10.1083/jcb.108.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eyken P., Sciot R., Desmet V. J. Expression of the novel extracellular matrix component tenascin in normal and diseased human liver. An immunohistochemical study. J Hepatol. 1990 Jul;11(1):43–52. doi: 10.1016/0168-8278(90)90270-2. [DOI] [PubMed] [Google Scholar]
- Vollmer G., Siegal G. P., Chiquet-Ehrismann R., Lightner V. A., Arnholdt H., Knuppen R. Tenascin expression in the human endometrium and in endometrial adenocarcinomas. Lab Invest. 1990 Jun;62(6):725–730. [PubMed] [Google Scholar]
- Yamada S., Ichida T., Matsuda Y., Miyazaki Y., Hatano T., Hata K., Asakura H., Hirota N., Geerts A., Wisse E. Tenascin expression in human chronic liver disease and in hepatocellular carcinoma. Liver. 1992 Feb;12(1):10–16. doi: 10.1111/j.1600-0676.1992.tb00548.x. [DOI] [PubMed] [Google Scholar]