Abstract
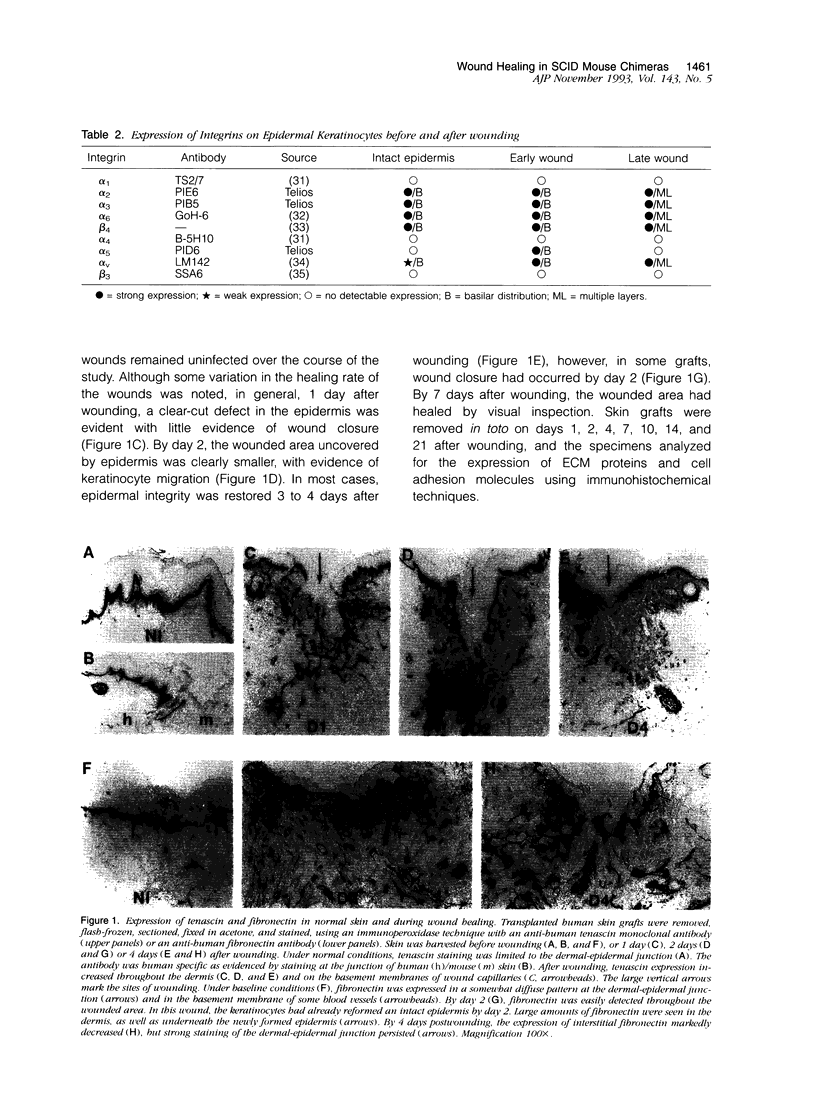
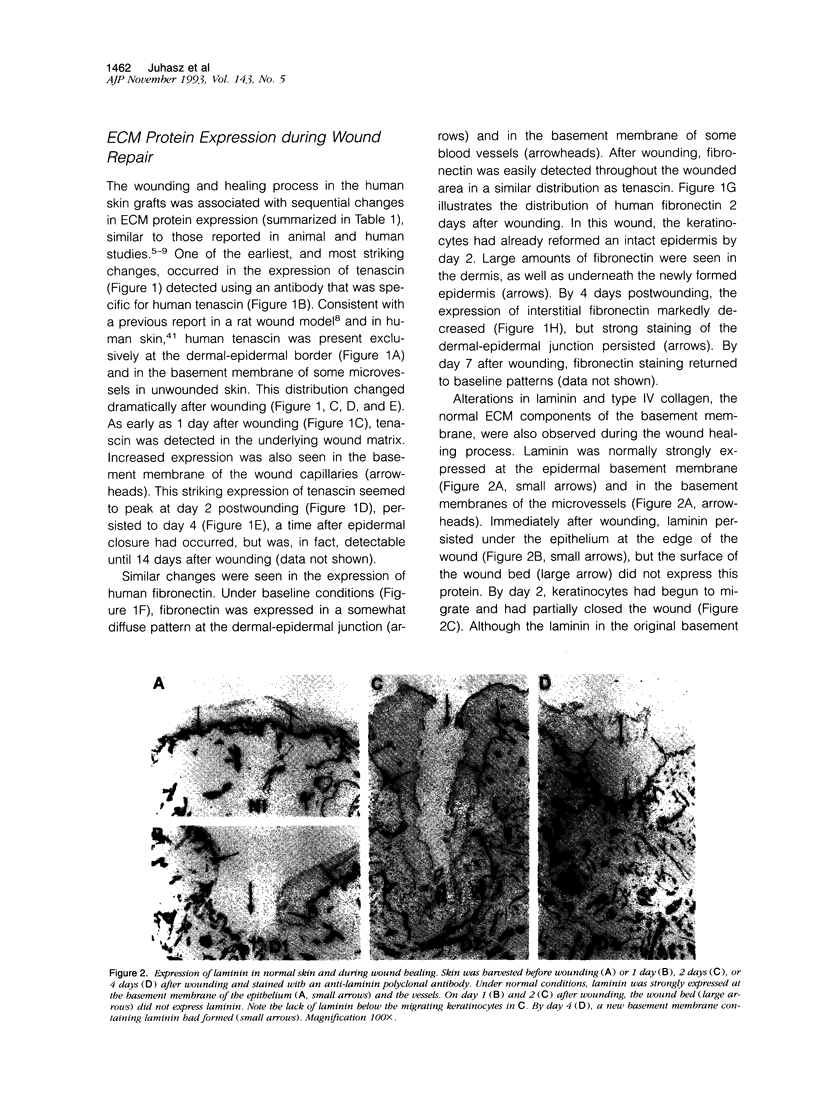
Although changes in extracellular matrix proteins during wound healing have been well documented, little is known about the regulation of corresponding extracellular matrix adhesion receptors (integrins). To study this process in a human in vivo model, full thickness human skin grafts were transplanted onto severe combined immunodeficient mice and deep excisional wounds involving both the epidermal and dermal layers were then made. The changes in the expression of cell matrix proteins and epithelial integrins over time were analyzed with specific antibodies using immunohistochemistry. Wounding was associated with alterations in extracellular matrix proteins, namely, loss of laminin and type IV collagen in the region of the wound and expression of tenascin and fibronectin. Changes were also noted in the integrins on the migrating keratinocytes. There was marked up-regulation of the alpha v subunit and de novo expression of the fibronectin receptor (alpha 5 beta 1) during the stage of active migration (days 1 to 3 after wounding). In the later stages of wound healing, after epithelial integrity had been established, redistribution of the alpha 2, alpha 3, alpha 6, and beta 4 collagen/laminin-binding integrin subunits to suprabasal epidermal layers was noted. Thus, during cutaneous wound healing, keratinocytes up-regulate fibronectin/fibrinogen-binding integrins and redistribute collagen/laminin-binding integrins. This study demonstrates that the human skin/severe combined immunodeficient chimera provides a useful model to study events during human wound repair.
Full text
PDF


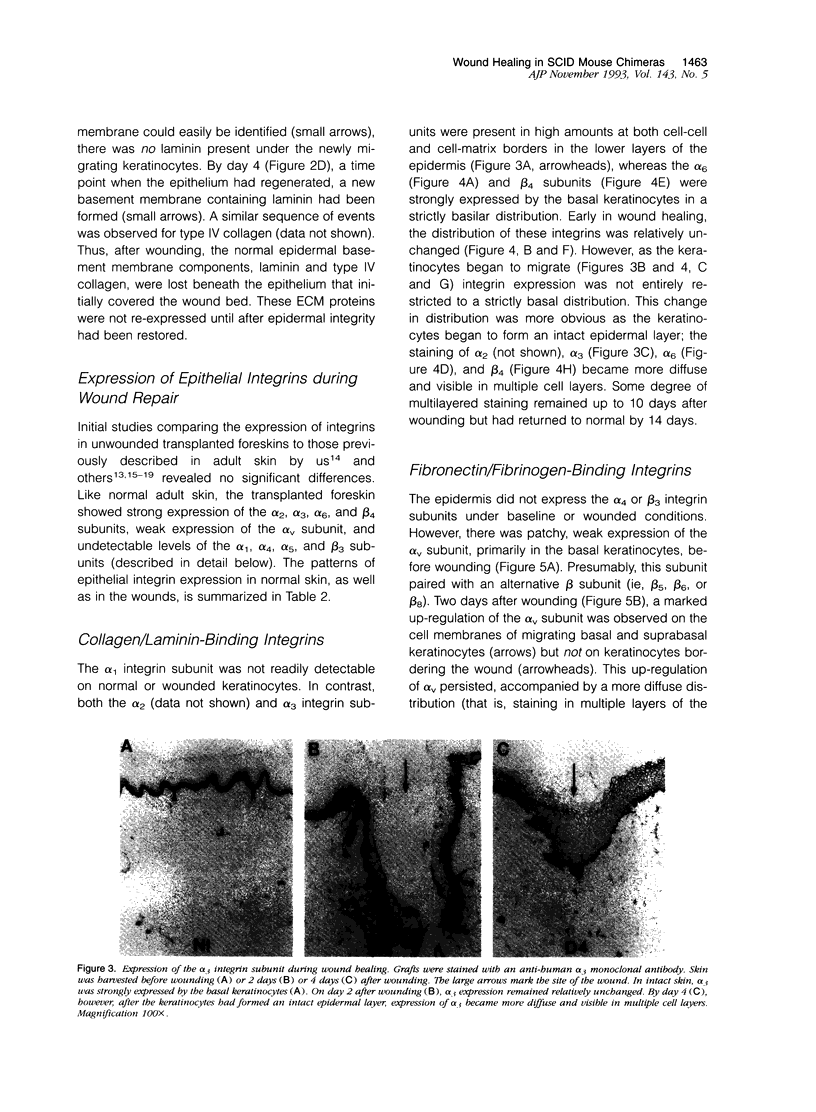
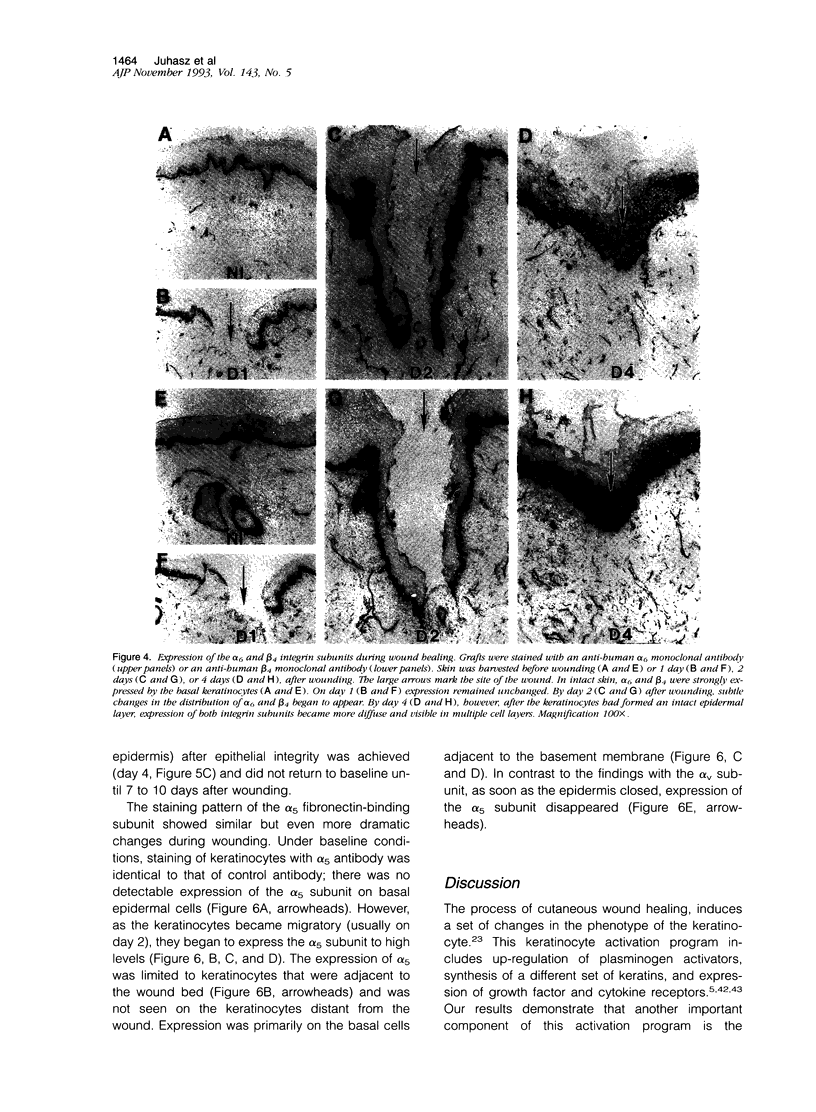





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Watt F. M. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell. 1990 Oct 19;63(2):425–435. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- Adams J. C., Watt F. M. Expression of beta 1, beta 3, beta 4, and beta 5 integrins by human epidermal keratinocytes and non-differentiating keratinocytes. J Cell Biol. 1991 Nov;115(3):829–841. doi: 10.1083/jcb.115.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Albelda S. M., Mette S. A., Elder D. E., Stewart R., Damjanovich L., Herlyn M., Buck C. A. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990 Oct 15;50(20):6757–6764. [PubMed] [Google Scholar]
- Albelda S. M., Muller W. A., Buck C. A., Newman P. J. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991 Sep;114(5):1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauvois B., Rouillard D., Sanceau J., Wietzerbin J. IFN-gamma and transforming growth factor-beta 1 differently regulate fibronectin and laminin receptors of human differentiating monocytic cells. J Immunol. 1992 Jun 15;148(12):3912–3919. [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Brass L. F., Shattil S. J., Kunicki T. J., Bennett J. S. Effect of calcium on the stability of the platelet membrane glycoprotein IIb-IIIa complex. J Biol Chem. 1985 Jul 5;260(13):7875–7881. [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Spiro R. C. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987 Dec 25;262(36):17703–17711. [PubMed] [Google Scholar]
- Clark R. A. Fibronectin matrix deposition and fibronectin receptor expression in healing and normal skin. J Invest Dermatol. 1990 Jun;94(6 Suppl):128S–134S. doi: 10.1111/1523-1747.ep12876104. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Lanigan J. M., DellaPelle P., Manseau E., Dvorak H. F., Colvin R. B. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J Invest Dermatol. 1982 Nov;79(5):264–269. doi: 10.1111/1523-1747.ep12500075. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Winn H. J., Dvorak H. F., Colvin R. B. Fibronectin beneath reepithelializing epidermis in vivo: sources and significance. J Invest Dermatol. 1983 Jun;80 (Suppl):26s–30s. [PubMed] [Google Scholar]
- Clark R. A. Wound repair. Curr Opin Cell Biol. 1989 Oct;1(5):1000–1008. doi: 10.1016/0955-0674(89)90072-0. [DOI] [PubMed] [Google Scholar]
- Cohen I. K., Mast B. A. Models of wound healing. J Trauma. 1990 Dec;30(12 Suppl):S149–S155. doi: 10.1097/00005373-199012001-00029. [DOI] [PubMed] [Google Scholar]
- Damjanovich L., Albelda S. M., Mette S. A., Buck C. A. Distribution of integrin cell adhesion receptors in normal and malignant lung tissue. Am J Respir Cell Mol Biol. 1992 Feb;6(2):197–206. doi: 10.1165/ajrcmb/6.2.197. [DOI] [PubMed] [Google Scholar]
- Demarchez M., Desbas C., Prunieras M. Wound healing of human skin transplanted on to the nude mouse. Br J Dermatol. 1985 Jul;113 (Suppl 28):177–182. doi: 10.1111/j.1365-2133.1985.tb15649.x. [DOI] [PubMed] [Google Scholar]
- Donaldson D. J., Mahan J. T. Fibrinogen and fibronectin as substrates for epidermal cell migration during wound closure. J Cell Sci. 1983 Jul;62:117–127. doi: 10.1242/jcs.62.1.117. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Van de Water L., Dvorak H. F., Hynes R. O. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989 Aug;109(2):903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F., Billingham R. E., Burgess L. Distribution of fibronectin during wound healing in vivo. J Invest Dermatol. 1981 Mar;76(3):181–189. doi: 10.1111/1523-1747.ep12525694. [DOI] [PubMed] [Google Scholar]
- Grinnell F. The activated keratinocyte: up regulation of cell adhesion and migration during wound healing. J Trauma. 1990 Dec;30(12 Suppl):S144–S149. [PubMed] [Google Scholar]
- Grinnell F. Wound repair, keratinocyte activation and integrin modulation. J Cell Sci. 1992 Jan;101(Pt 1):1–5. doi: 10.1242/jcs.101.1.1. [DOI] [PubMed] [Google Scholar]
- Grøndahl-Hansen J., Lund L. R., Ralfkiaer E., Ottevanger V., Danø K. Urokinase- and tissue-type plasminogen activators in keratinocytes during wound reepithelialization in vivo. J Invest Dermatol. 1988 Jun;90(6):790–795. doi: 10.1111/1523-1747.ep12461511. [DOI] [PubMed] [Google Scholar]
- Guo M., Kim L. T., Akiyama S. K., Gralnick H. R., Yamada K. M., Grinnell F. Altered processing of integrin receptors during keratinocyte activation. Exp Cell Res. 1991 Aug;195(2):315–322. doi: 10.1016/0014-4827(91)90379-9. [DOI] [PubMed] [Google Scholar]
- Guo M., Toda K., Grinnell F. Activation of human keratinocyte migration on type I collagen and fibronectin. J Cell Sci. 1990 Jun;96(Pt 2):197–205. doi: 10.1242/jcs.96.2.197. [DOI] [PubMed] [Google Scholar]
- Heino J., Ignotz R. A., Hemler M. E., Crouse C., Massagué J. Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J Biol Chem. 1989 Jan 5;264(1):380–388. [PubMed] [Google Scholar]
- Heino J., Massagué J. Transforming growth factor-beta switches the pattern of integrins expressed in MG-63 human osteosarcoma cells and causes a selective loss of cell adhesion to laminin. J Biol Chem. 1989 Dec 25;264(36):21806–21811. [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Herlyn M., Graeven U., Speicher D., Sela B. A., Bennicelli J. L., Kath R., Guerry D., 4th Characterization of tenascin secreted by human melanoma cells. Cancer Res. 1991 Sep 15;51(18):4853–4858. [PubMed] [Google Scholar]
- Hertle M. D., Adams J. C., Watt F. M. Integrin expression during human epidermal development in vivo and in vitro. Development. 1991 May;112(1):193–206. doi: 10.1242/dev.112.1.193. [DOI] [PubMed] [Google Scholar]
- Hertle M. D., Kubler M. D., Leigh I. M., Watt F. M. Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J Clin Invest. 1992 Jun;89(6):1892–1901. doi: 10.1172/JCI115794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne R. S., Hurley J. V., Crowe D. M., Ritz M., O'Brien B. M., Arnold L. I. Wound healing in foetal sheep: a histological and electron microscope study. Br J Plast Surg. 1992 Jul;45(5):333–344. doi: 10.1016/0007-1226(92)90001-e. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Cell adhesion protein receptors as targets for transforming growth factor-beta action. Cell. 1987 Oct 23;51(2):189–197. doi: 10.1016/0092-8674(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Juhasz I., Albelda S. M., Elder D. E., Murphy G. F., Adachi K., Herlyn D., Valyi-Nagy I. T., Herlyn M. Growth and invasion of human melanomas in human skin grafted to immunodeficient mice. Am J Pathol. 1993 Aug;143(2):528–537. [PMC free article] [PubMed] [Google Scholar]
- Kellner I., Konter U., Sterry W. Overexpression of extracellular matrix receptors (VLA-3, 5 and 6) on psoriatic keratinocytes. Br J Dermatol. 1991 Sep;125(3):211–216. doi: 10.1111/j.1365-2133.1991.tb14742.x. [DOI] [PubMed] [Google Scholar]
- Kennel S. J., Foote L. J., Falcioni R., Sonnenberg A., Stringer C. D., Crouse C., Hemler M. E. Analysis of the tumor-associated antigen TSP-180. Identity with alpha 6-beta 4 in the integrin superfamily. J Biol Chem. 1989 Sep 15;264(26):15515–15521. [PubMed] [Google Scholar]
- Kim L. T., Ishihara S., Lee C. C., Akiyama S. K., Yamada K. M., Grinnell F. Altered glycosylation and cell surface expression of beta 1 integrin receptors during keratinocyte activation. J Cell Sci. 1992 Nov;103(Pt 3):743–753. doi: 10.1242/jcs.103.3.743. [DOI] [PubMed] [Google Scholar]
- Klein C. E., Steinmayer T., Mattes J. M., Kaufmann R., Weber L. Integrins of normal human epidermis: differential expression, synthesis and molecular structure. Br J Dermatol. 1990 Aug;123(2):171–178. doi: 10.1111/j.1365-2133.1990.tb01844.x. [DOI] [PubMed] [Google Scholar]
- Koukoulis G. K., Gould V. E., Bhattacharyya A., Gould J. E., Howeedy A. A., Virtanen I. Tenascin in normal, reactive, hyperplastic, and neoplastic tissues: biologic and pathologic implications. Hum Pathol. 1991 Jul;22(7):636–643. doi: 10.1016/0046-8177(91)90285-w. [DOI] [PubMed] [Google Scholar]
- Mackie E. J., Halfter W., Liverani D. Induction of tenascin in healing wounds. J Cell Biol. 1988 Dec;107(6 Pt 2):2757–2767. doi: 10.1083/jcb.107.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Roll F. J., Furthmayr H., Foidart J. M. Ultrastructural localization of fibronectin and laminin in the basement membranes of the murine kidney. J Cell Biol. 1980 Aug;86(2):682–687. doi: 10.1083/jcb.86.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff N. T., Lowrey C., Decker C., Tovar A., Damsky C., Buck C., Horwitz A. F. A monoclonal antibody detaches embryonic skeletal muscle from extracellular matrices. J Cell Biol. 1982 Nov;95(2 Pt 1):654–666. doi: 10.1083/jcb.95.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T., Nakagawa S., Awata T., Ohashi Y., Watanabe K., Manabe R. Fibronectin promotes epithelial migration of cultured rabbit cornea in situ. J Cell Biol. 1983 Nov;97(5 Pt 1):1653–1657. doi: 10.1083/jcb.97.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe E. J., Payne R. E., Jr, Russell N., Woodley D. T. Spreading and enhanced motility of human keratinocytes on fibronectin. J Invest Dermatol. 1985 Aug;85(2):125–130. doi: 10.1111/1523-1747.ep12276531. [DOI] [PubMed] [Google Scholar]
- Pellegrini G., De Luca M., Orecchia G., Balzac F., Cremona O., Savoia P., Cancedda R., Marchisio P. C. Expression, topography, and function of integrin receptors are severely altered in keratinocytes from involved and uninvolved psoriatic skin. J Clin Invest. 1992 Jun;89(6):1783–1795. doi: 10.1172/JCI115782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen J., Larjava H., Jaakkola S., Gralnick H., Akiyama S. K., Yamada S. S., Yamada K. M., Uitto J. Localization of integrin receptors for fibronectin, collagen, and laminin in human skin. Variable expression in basal and squamous cell carcinomas. J Clin Invest. 1989 Dec;84(6):1916–1923. doi: 10.1172/JCI114379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. J., Birkenmeier T. M., McQuillan J. J., Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M., McDonald J. A. Transforming growth factor beta stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J Biol Chem. 1988 Apr 5;263(10):4586–4592. [PubMed] [Google Scholar]
- Santala P., Heino J. Regulation of integrin-type cell adhesion receptors by cytokines. J Biol Chem. 1991 Dec 5;266(34):23505–23509. [PubMed] [Google Scholar]
- Schalkwijk J., Steijlen P. M., van Vlijmen-Willems I. M., Oosterling B., Mackie E. J., Verstraeten A. A. Tenascin expression in human dermis is related to epidermal proliferation. Am J Pathol. 1991 Nov;139(5):1143–1150. [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., Modderman P. W., Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988 Dec 1;336(6198):487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Alvarez O. M., Bere E. W., Jr, Eaglstein W. H., Katz S. I. Detection of basement membrane zone antigens during epidermal wound healing in pigs. J Invest Dermatol. 1981 Aug;77(2):240–243. doi: 10.1111/1523-1747.ep12480082. [DOI] [PubMed] [Google Scholar]
- Stepp M. A., Spurr-Michaud S., Gipson I. K. Integrins in the wounded and unwounded stratified squamous epithelium of the cornea. Invest Ophthalmol Vis Sci. 1993 Apr;34(5):1829–1844. [PubMed] [Google Scholar]
- Streuli C. H., Schmidhauser C., Kobrin M., Bissell M. J., Derynck R. Extracellular matrix regulates expression of the TGF-beta 1 gene. J Cell Biol. 1993 Jan;120(1):253–260. doi: 10.1083/jcb.120.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A., Billingham R. E., Grinnell F. Activation of rabbit keratinocyte fibronectin receptor function in vivo during wound healing. J Invest Dermatol. 1986 May;86(5):585–590. doi: 10.1111/1523-1747.ep12355243. [DOI] [PubMed] [Google Scholar]
- Toda K., Tuan T. L., Brown P. J., Grinnell F. Fibronectin receptors of human keratinocytes and their expression during cell culture. J Cell Biol. 1987 Dec;105(6 Pt 2):3097–3104. doi: 10.1083/jcb.105.6.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenczak B. A., Lynch J. B., Nanney L. B. Epidermal growth factor receptor distribution in burn wounds. Implications for growth factor-mediated repair. J Clin Invest. 1992 Dec;90(6):2392–2401. doi: 10.1172/JCI116130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley D. T., Bachmann P. M., O'Keefe E. J. Laminin inhibits human keratinocyte migration. J Cell Physiol. 1988 Jul;136(1):140–146. doi: 10.1002/jcp.1041360118. [DOI] [PubMed] [Google Scholar]
- Woodley D. T., O'Keefe E. J., Prunieras M. Cutaneous wound healing: a model for cell-matrix interactions. J Am Acad Dermatol. 1985 Feb;12(2 Pt 2):420–433. doi: 10.1016/s0190-9622(85)80005-0. [DOI] [PubMed] [Google Scholar]
- Yan H. C., Juhasz I., Pilewski J., Murphy G. F., Herlyn M., Albelda S. M. Human/severe combined immunodeficient mouse chimeras. An experimental in vivo model system to study the regulation of human endothelial cell-leukocyte adhesion molecules. J Clin Invest. 1993 Mar;91(3):986–996. doi: 10.1172/JCI116320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambruno G., Manca V., Santantonio M. L., Soligo D., Giannetti A. VLA protein expression on epidermal cells (keratinocytes, Langerhans cells, melanocytes): a light and electron microscopic immunohistochemical study. Br J Dermatol. 1991 Feb;124(2):135–145. doi: 10.1111/j.1365-2133.1991.tb00422.x. [DOI] [PubMed] [Google Scholar]