Abstract
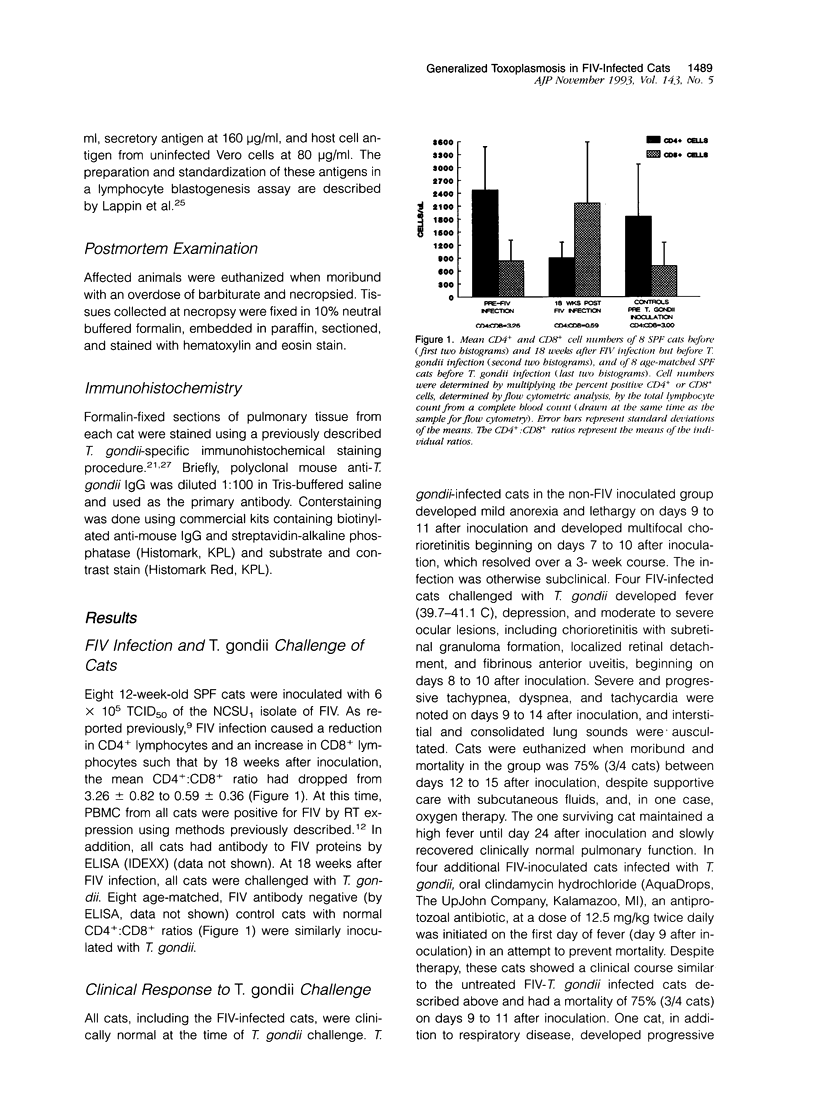
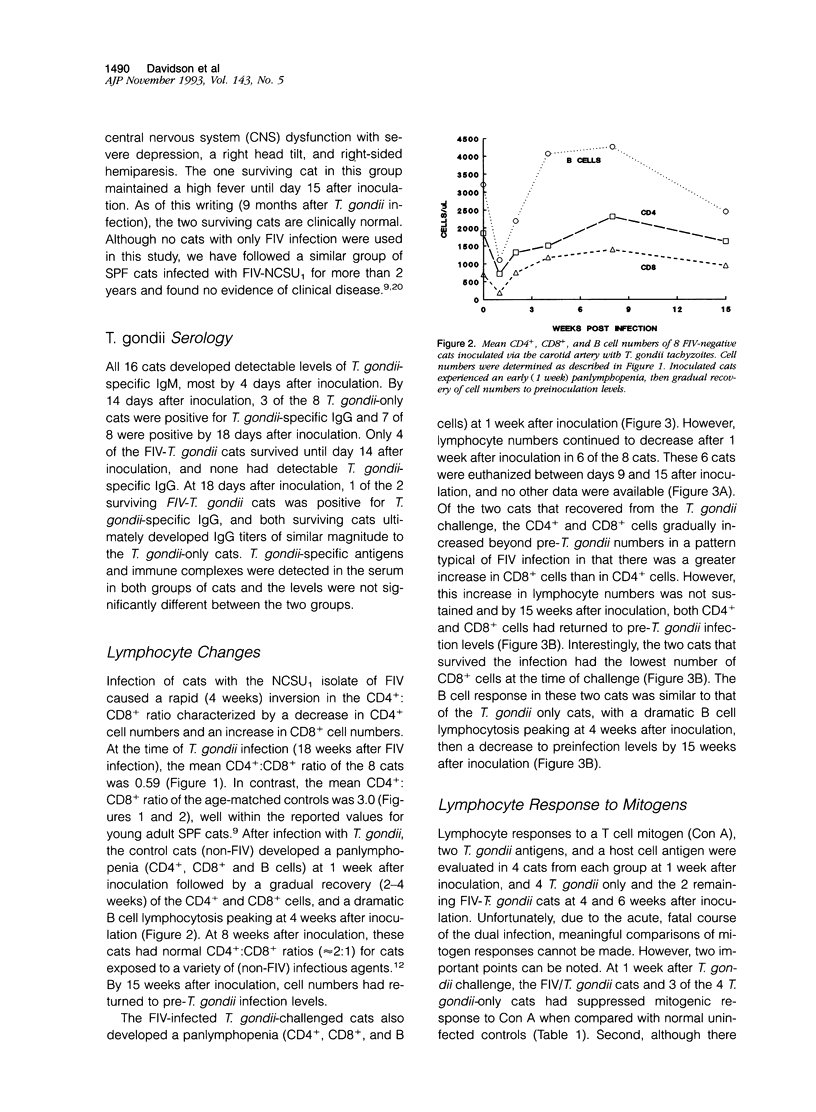
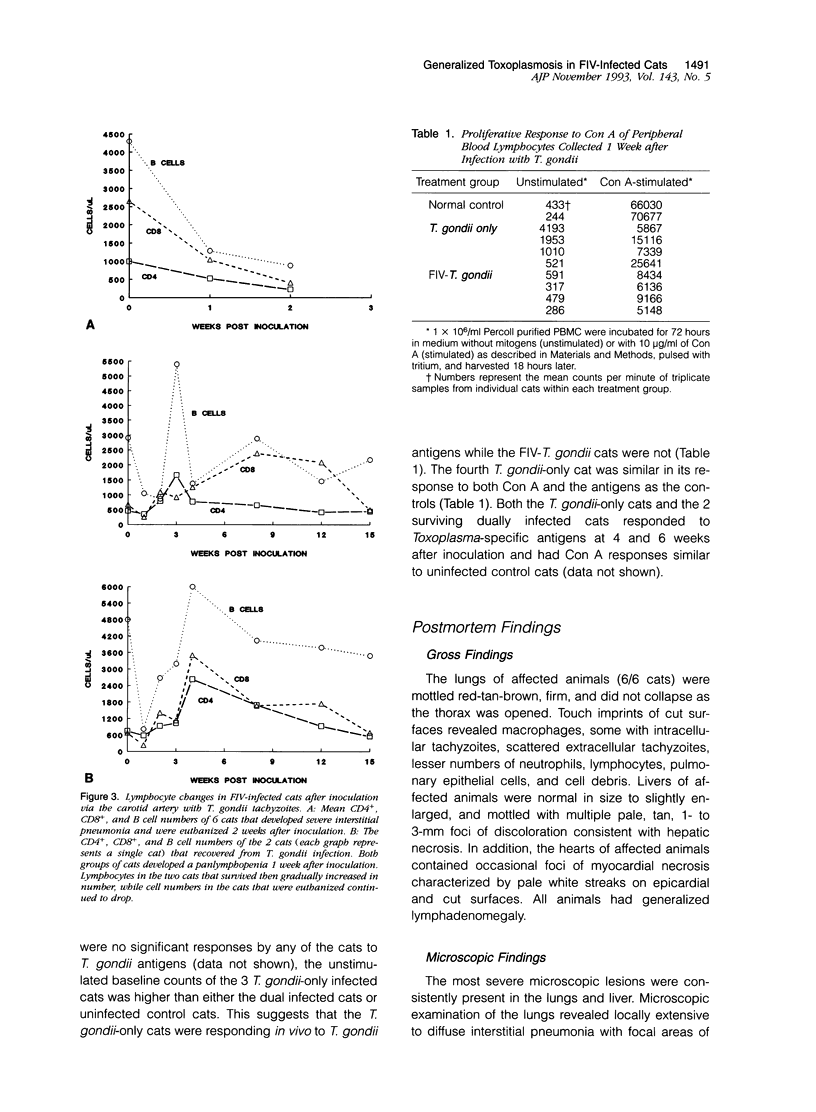
This study was designed to examine the effects of a pre-existing, clinically asymptomatic feline immunodeficiency virus (FIV) infection on a primary challenge with Toxoplasma gondii. Parenteral challenge of FIV-infected cats with tachyzoites of the ME49 strain of T. gondii caused a precipitous drop in all lymphocytes (CD4+, CD8+, and B cells) and generalized severe toxoplasmosis. The predominant postmortem lesions included acute and often fatal interstitial pneumonia, dominated histologically by macrophages, and multifocal to coalescing hepatic necrosis. Immunohistochemistry revealed numerous T. gondii antigen and tachyzoites in macrophages and other cell types in the lung lesions. The proliferative response of peripheral blood mononuclear cells to specific (T. gondii antigen) and nonspecific (Concanavalin A) mitogens was defective in the dually infected cats, suggesting marked immunosuppression. In contrast to the dually infected cats, cats infected only with T. gondii developed a transient, mild clinical disease characterized by anorexia, lethargy, and multifocal chorioretinitis. Lymphocyte changes in T. gondii-infected cats included an early pan-lymphopenia followed by reestablishment of all lymphocyte subset profiles. These cats also showed a reduced proliferative response to Concanavalin A at 1 week after challenge, but a measurable in vivo response to T. gondii antigens, as evidenced by in vitro lymphocyte proliferation in the absence of a mitogenic stimulus. These results show that infection of cats with FIV-NCSU, markedly enhances their susceptibility to a primary T. gondii infection and provides a model to study the mechanisms of the underlying immunological defect(s) occurring early after HIV infection that may predispose individuals to development of acquired immunodeficiency syndrome and associated diseases.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackley C. D., Yamamoto J. K., Levy N., Pedersen N. C., Cooper M. D. Immunologic abnormalities in pathogen-free cats experimentally infected with feline immunodeficiency virus. J Virol. 1990 Nov;64(11):5652–5655. doi: 10.1128/jvi.64.11.5652-5655.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G. Depletion of L3T4+ (CD4+) T lymphocytes prevents development of resistance to Toxoplasma gondii in mice. Infect Immun. 1991 May;59(5):1614–1619. doi: 10.1128/iai.59.5.1614-1619.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlough J. E., Ackley C. D., George J. W., Levy N., Acevedo R., Moore P. F., Rideout B. A., Cooper M. D., Pedersen N. C. Acquired immune dysfunction in cats with experimentally induced feline immunodeficiency virus infection: comparison of short-term and long-term infections. J Acquir Immune Defic Syndr. 1991;4(3):219–227. [PubMed] [Google Scholar]
- Black C. M., Catterall J. R., Remington J. S. In vivo and in vitro activation of alveolar macrophages by recombinant interferon-gamma. J Immunol. 1987 Jan 15;138(2):491–495. [PubMed] [Google Scholar]
- Black C. M., Israelski D. M., Suzuki Y., Remington J. S. Effect of recombinant tumour necrosis factor on acute infection in mice with Toxoplasma gondii or Trypanosoma cruzi. Immunology. 1989 Dec;68(4):570–574. [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Stocks N. I., Zajac R. A., Boswell R. N., Lucey D. R., Via C. S., Shearer G. M. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989 Dec;84(6):1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe S. M., Carlin J. B., Stewart K. I., Lucas C. R., Hoy J. F. Predictive value of CD4 lymphocyte numbers for the development of opportunistic infections and malignancies in HIV-infected persons. J Acquir Immune Defic Syndr. 1991;4(8):770–776. [PubMed] [Google Scholar]
- Dubey J. P., Frenkel J. K. Immunity to feline toxoplasmosis: modification by administration of corticosteroids. Vet Pathol. 1974;11(4):350–379. doi: 10.1177/030098587401100407. [DOI] [PubMed] [Google Scholar]
- Duquesne V., Auriault C., Darcy F., Decavel J. P., Capron A. Protection of nude rats against Toxoplasma infection by excreted-secreted antigen-specific helper T cells. Infect Immun. 1990 Jul;58(7):2120–2126. doi: 10.1128/iai.58.7.2120-2126.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English R. V., Davidson M. G., Nasisse M. P., Jamieson V. E., Lappin M. R. Intraocular disease associated with feline immunodeficiency virus infection in cats. J Am Vet Med Assoc. 1990 Apr 1;196(7):1116–1119. [PubMed] [Google Scholar]
- Frenkel J. K. Pathophysiology of toxoplasmosis. Parasitol Today. 1988 Oct;4(10):273–278. doi: 10.1016/0169-4758(88)90018-x. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R. T., Hartley J. W., Fredrickson T. N., Chattopadhyay S. K., Sher A., Morse H. C., 3rd Opportunistic infections and retrovirus-induced immunodeficiency: studies of acute and chronic infections with Toxoplasma gondii in mice infected with LP-BM5 murine leukemia viruses. Infect Immun. 1992 Oct;60(10):4394–4401. doi: 10.1128/iai.60.10.4394-4401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. L. SCID mouse models of acute and relapsing chronic Toxoplasma gondii infections. Infect Immun. 1992 Sep;60(9):3719–3724. doi: 10.1128/iai.60.9.3719-3724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin M. R., Cayatte S., Powell C. C., Gigliotti A., Cooper C., Roberts S. M. Detection of Toxoplasma gondii antigen-containing immune complexes in the serum of cats. Am J Vet Res. 1993 Mar;54(3):415–419. [PubMed] [Google Scholar]
- Lappin M. R., Dawe D. L., Lindl P., Greene C. E., Prestwood A. K. Mitogen and antigen-specific induction of lymphoblast transformation in cats with subclinical toxoplasmosis. Vet Immunol Immunopathol. 1992 Jan 15;30(2-3):207–220. doi: 10.1016/0165-2427(92)90139-h. [DOI] [PubMed] [Google Scholar]
- Lappin M. R., Greene C. E., Prestwood A. K., Dawe D. L., Tarleton R. L. Diagnosis of recent Toxoplasma gondii infection in cats by use of an enzyme-linked immunosorbent assay for immunoglobulin M. Am J Vet Res. 1989 Sep;50(9):1580–1585. [PubMed] [Google Scholar]
- Lappin M. R., Greene C. E., Prestwood A. K., Dawe D. L., Tarleton R. L. Enzyme-linked immunosorbent assay for the detection of circulating antigens of Toxoplasma gondii in the serum of cats. Am J Vet Res. 1989 Sep;50(9):1586–1590. [PubMed] [Google Scholar]
- Lappin M. R., Greene C. E., Winston S., Toll S. L., Epstein M. E. Clinical feline toxoplasmosis. Serologic diagnosis and therapeutic management of 15 cases. J Vet Intern Med. 1989 Jul-Sep;3(3):139–143. doi: 10.1111/j.1939-1676.1989.tb03089.x. [DOI] [PubMed] [Google Scholar]
- Lin D. S., Bowman D. D., Jacobson R. H. Immunological changes in cats with concurrent Toxoplasma gondii and feline immunodeficiency virus infections. J Clin Microbiol. 1992 Jan;30(1):17–24. doi: 10.1128/jcm.30.1.17-24.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg R. E., Frenkel J. K. Toxoplasmosis in nude mice. J Parasitol. 1977 Apr;63(2):219–221. [PubMed] [Google Scholar]
- Luft B. J., Brooks R. G., Conley F. K., McCabe R. E., Remington J. S. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA. 1984 Aug 17;252(7):913–917. [PubMed] [Google Scholar]
- Luft B. J., Remington J. S. AIDS commentary. Toxoplasmic encephalitis. J Infect Dis. 1988 Jan;157(1):1–6. doi: 10.1093/infdis/157.1.1. [DOI] [PubMed] [Google Scholar]
- McChesney M. B., Oldstone M. B. Virus-induced immunosuppression: infections with measles virus and human immunodeficiency virus. Adv Immunol. 1989;45:335–380. doi: 10.1016/s0065-2776(08)60696-3. [DOI] [PubMed] [Google Scholar]
- Novotney C., English R. V., Housman J., Davidson M. G., Nasisse M. P., Jeng C. R., Davis W. C., Tompkins M. B. Lymphocyte population changes in cats naturally infected with feline immunodeficiency virus. AIDS. 1990 Dec;4(12):1213–1218. doi: 10.1097/00002030-199012000-00005. [DOI] [PubMed] [Google Scholar]
- Parker G. A., Langloss J. M., Dubey J. P., Hoover E. A. Pathogenesis of acute toxoplasmosis in specific-pathogen-free cats. Vet Pathol. 1981 Nov;18(6):786–803. doi: 10.1177/030098588101800609. [DOI] [PubMed] [Google Scholar]
- Pavia C. S. Protection against experimental toxoplasmosis by adoptive immunotherapy. J Immunol. 1986 Nov 1;137(9):2985–2990. [PubMed] [Google Scholar]
- Pedersen N. C., Yamamoto J. K., Ishida T., Hansen H. Feline immunodeficiency virus infection. Vet Immunol Immunopathol. 1989 May;21(1):111–129. doi: 10.1016/0165-2427(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Rojko J. L., Hoover E. A., Mathes L. E., Olsen R. G., Schaller J. P. Pathogenesis of experimental feline leukemia virus infection. J Natl Cancer Inst. 1979 Sep;63(3):759–768. doi: 10.1093/jnci/63.3.759. [DOI] [PubMed] [Google Scholar]
- Sibley L. D., Adams L. B., Fukutomi Y., Krahenbuhl J. L. Tumor necrosis factor-alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J Immunol. 1991 Oct 1;147(7):2340–2345. [PubMed] [Google Scholar]
- Siebelink K. H., Chu I. H., Rimmelzwaan G. F., Weijer K., van Herwijnen R., Knell P., Egberink H. F., Bosch M. L., Osterhaus A. D. Feline immunodeficiency virus (FIV) infection in the cat as a model for HIV infection in man: FIV-induced impairment of immune function. AIDS Res Hum Retroviruses. 1990 Dec;6(12):1373–1378. doi: 10.1089/aid.1990.6.1373. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Orellana M. A., Schreiber R. D., Remington J. S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988 Apr 22;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Remington J. S. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J Immunol. 1988 Jun 1;140(11):3943–3946. [PubMed] [Google Scholar]
- Suzuki Y., Remington J. S. The effect of anti-IFN-gamma antibody on the protective effect of Lyt-2+ immune T cells against toxoplasmosis in mice. J Immunol. 1990 Mar 1;144(5):1954–1956. [PubMed] [Google Scholar]
- Tompkins M. B., Gebhard D. H., Bingham H. R., Hamilton M. J., Davis W. C., Tompkins W. A. Characterization of monoclonal antibodies to feline T lymphocytes and their use in the analysis of lymphocyte tissue distribution in the cat. Vet Immunol Immunopathol. 1990 Dec;26(4):305–317. doi: 10.1016/0165-2427(90)90115-9. [DOI] [PubMed] [Google Scholar]
- Tompkins M. B., Nelson P. D., English R. V., Novotney C. Early events in the immunopathogenesis of feline retrovirus infections. J Am Vet Med Assoc. 1991 Nov 15;199(10):1311–1315. [PubMed] [Google Scholar]
- Tompkins M. B., Ogilvie G. K., Franklin R. A., Kelley K. W., Tompkins W. A. Induction of IL-2 and lymphokine activated killer cells in the cat. Vet Immunol Immunopathol. 1987 Sep;16(1-2):1–10. doi: 10.1016/0165-2427(87)90169-3. [DOI] [PubMed] [Google Scholar]
- Torten M., Franchini M., Barlough J. E., George J. W., Mozes E., Lutz H., Pedersen N. C. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J Virol. 1991 May;65(5):2225–2230. doi: 10.1128/jvi.65.5.2225-2230.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla A., Sjöland L., Dubey J. P. Immunohistochemical diagnosis of toxoplasmosis in fetuses and fetal membranes of sheep. Am J Vet Res. 1987 Mar;48(3):348–351. [PubMed] [Google Scholar]
- Vollmer T. L., Waldor M. K., Steinman L., Conley F. K. Depletion of T-4+ lymphocytes with monoclonal antibody reactivates toxoplasmosis in the central nervous system: a model of superinfection in AIDS. J Immunol. 1987 Jun 1;138(11):3737–3741. [PubMed] [Google Scholar]
- Walker C. M., Erickson A. L., Hsueh F. C., Levy J. A. Inhibition of human immunodeficiency virus replication in acutely infected CD4+ cells by CD8+ cells involves a noncytotoxic mechanism. J Virol. 1991 Nov;65(11):5921–5927. doi: 10.1128/jvi.65.11.5921-5927.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. M., Moody D. J., Stites D. P., Levy J. A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986 Dec 19;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Weimer R., Schweighoffer T., Schimpf K., Opelz G. Helper and suppressor T-cell function in HIV-infected hemophilia patients. Blood. 1989 Jul;74(1):298–302. [PubMed] [Google Scholar]
- Yamamoto J. K., Hansen H., Ho E. W., Morishita T. Y., Okuda T., Sawa T. R., Nakamura R. M., Pedersen N. C. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J Am Vet Med Assoc. 1989 Jan 15;194(2):213–220. [PubMed] [Google Scholar]
- Yamamoto J. K., Sparger E., Ho E. W., Andersen P. R., O'Connor T. P., Mandell C. P., Lowenstine L., Munn R., Pedersen N. C. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988 Aug;49(8):1246–1258. [PubMed] [Google Scholar]



