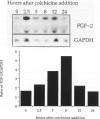
Abstract
Cultured endothelial cells characteristically form a monolayer and become quiescent at saturation density. This study shows that microtubule destabilization in confluent cultures of bovine aortic endothelial cells stimulates fibroblast growth factor 2 (FGF-2, bFGF)-dependent DNA synthesis. Twenty-four hours after addition of the microtubule-disrupting drug colchicine, tritiated thymidine incorporation increases up to fivefold when compared to control cultures. Significant stimulation is seen with doses from 0.05 to 1.0 microgram/ml. The effect of colchicine is quantitatively similar to stimulation of the same cultures with 5 ng/ml FGF-2. Furthermore, the stimulation of DNA synthesis by colchicine can be completely blocked by the addition of a neutralizing antibody to FGF-2. This suggests that colchicine may stimulate bovine aortic endothelial cells by modulating endogenous FGF-2/receptor interactions or that colchicine acts by a different mechanism that requires the growth factor for mitogenicity. The combined effects of colchicine and FGF-2 are more than additive, which supports the idea that microtubule disruption may facilitate cellular response to FGF-2. Cytochalasin B, preventing actin polymerization, inhibits the mitogenic response to FGF-2 but not the response to colchicine. These results are best interpreted as evidence that colchicine stimulates endothelial cell DNA synthesis by a pathway that requires endogenous FGF-2 and may be facilitating cellular responsiveness to the growth factor by disrupting the monolayer via the cytoskeleton.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Bavisotto L. M., Schwartz S. M., Heimark R. L. Modulation of Ca2(+)-dependent intercellular adhesion in bovine aortic and human umbilical vein endothelial cells by heparin-binding growth factors. J Cell Physiol. 1990 Apr;143(1):39–51. doi: 10.1002/jcp.1041430106. [DOI] [PubMed] [Google Scholar]
- Brigstock D. R., Klagsbrun M., Sasse J., Farber P. A., Iberg N. Species-specific high molecular weight forms of basic fibroblast growth factor. Growth Factors. 1990;4(1):45–52. doi: 10.3109/08977199009011009. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Friedkin M., Rozengurt E. Colchicine inhibits epidermal growth factor degradation in 3T3 cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):480–484. doi: 10.1073/pnas.77.1.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin K. L., Carney D. H. Evidence that microtubule depolymerization early in the cell cycle is sufficient to initiate DNA synthesis. Cell. 1981 Jan;23(1):61–71. doi: 10.1016/0092-8674(81)90270-1. [DOI] [PubMed] [Google Scholar]
- Florkiewicz R. Z., Baird A., Gonzalez A. M. Multiple forms of bFGF: differential nuclear and cell surface localization. Growth Factors. 1991;4(4):265–275. doi: 10.3109/08977199109043912. [DOI] [PubMed] [Google Scholar]
- Friedkin M., Legg A., Rozengurt E. Antitubulin agents enhance the stimulation of DNA synthesis by polypeptide growth factors in 3T3 mouse fibroblasts. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3909–3912. doi: 10.1073/pnas.76.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek C. M., Carbon S. Injury-induced release of basic fibroblast growth factor from bovine aortic endothelium. J Cell Physiol. 1989 Jun;139(3):570–579. doi: 10.1002/jcp.1041390317. [DOI] [PubMed] [Google Scholar]
- Gajdusek C. M., Schwartz S. M. Technique for cloning bovine aortic endothelial cells. In Vitro. 1983 May;19(5):394–402. doi: 10.1007/BF02619556. [DOI] [PubMed] [Google Scholar]
- Geiger B., Avnur Z., Schlessinger J. Restricted mobility of membrane constituents in cell-substrate focal contacts of chicken fibroblasts. J Cell Biol. 1982 May;93(2):495–500. doi: 10.1083/jcb.93.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Expression and control of vascular endothelial cells: proliferation and differentiation by fibroblast growth factors. J Invest Dermatol. 1989 Aug;93(2 Suppl):39S–47S. doi: 10.1111/1523-1747.ep12580907. [DOI] [PubMed] [Google Scholar]
- Grothe C., Zachmann K., Unsicker K., Westermann R. High molecular weight forms of basic fibroblast growth factor recognized by a new anti-bFGF antibody. FEBS Lett. 1990 Jan 15;260(1):35–38. doi: 10.1016/0014-5793(90)80059-r. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Chao S., Schwartz S. M., Reidy M. A. Aortic endothelial cell death and replication in normal and lipopolysaccharide-treated rats. Am J Pathol. 1985 Oct;121(1):123–127. [PMC free article] [PubMed] [Google Scholar]
- Heimark R. L., Schwartz S. M. The role of membrane-membrane interactions in the regulation of endothelial cell growth. J Cell Biol. 1985 Jun;100(6):1934–1940. doi: 10.1083/jcb.100.6.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci U S A. 1990 May;87(9):3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E., Madri J. A., Folkman J. Endothelial growth factors and extracellular matrix regulate DNA synthesis through modulation of cell and nuclear expansion. In Vitro Cell Dev Biol. 1987 May;23(5):387–394. doi: 10.1007/BF02620997. [DOI] [PubMed] [Google Scholar]
- Ingber D. Extracellular matrix and cell shape: potential control points for inhibition of angiogenesis. J Cell Biochem. 1991 Nov;47(3):236–241. doi: 10.1002/jcb.240470309. [DOI] [PubMed] [Google Scholar]
- Kardami E., Stoski R. M., Doble B. W., Yamamoto T., Hertzberg E. L., Nagy J. I. Biochemical and ultrastructural evidence for the association of basic fibroblast growth factor with cardiac gap junctions. J Biol Chem. 1991 Oct 15;266(29):19551–19557. [PubMed] [Google Scholar]
- Lindner V., Majack R. A., Reidy M. A. Basic fibroblast growth factor stimulates endothelial regrowth and proliferation in denuded arteries. J Clin Invest. 1990 Jun;85(6):2004–2008. doi: 10.1172/JCI114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner V., Reidy M. A. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier J. A., Rusnati M., Ragnotti G., Presta M. Characterization of a Mr 20,000 basic fibroblast growth factor-like protein secreted by normal and transformed fetal bovine aortic endothelial cells. Exp Cell Res. 1990 Feb;186(2):354–361. doi: 10.1016/0014-4827(90)90316-3. [DOI] [PubMed] [Google Scholar]
- McNeil P. L., Muthukrishnan L., Warder E., D'Amore P. A. Growth factors are released by mechanically wounded endothelial cells. J Cell Biol. 1989 Aug;109(2):811–822. doi: 10.1083/jcb.109.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukrishnan L., Warder E., McNeil P. L. Basic fibroblast growth factor is efficiently released from a cytolsolic storage site through plasma membrane disruptions of endothelial cells. J Cell Physiol. 1991 Jul;148(1):1–16. doi: 10.1002/jcp.1041480102. [DOI] [PubMed] [Google Scholar]
- Otto A. M., Zumbé A., Gibson L., Kubler A. M., Jimenez de Asua L. Cytoskeleton-disrupting drugs enhance effect of growth factors and hormones on initiation of DNA synthesis. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6435–6438. doi: 10.1073/pnas.76.12.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesanti E. L., Axline S. G. Colchicine effects on lysosomal enzyme induction and intracellular degradation in the cultivated macrophage. J Exp Med. 1975 May 1;141(5):1030–1046. doi: 10.1084/jem.141.5.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly T. M., Taylor D. S., Herblin W. F., Thoolen M. J., Chiu A. T., Watson D. W., Timmermans P. B. Monoclonal antibodies directed against basic fibroblast growth factor which inhibit its biological activity in vitro and in vivo. Biochem Biophys Res Commun. 1989 Oct 31;164(2):736–743. doi: 10.1016/0006-291x(89)91521-0. [DOI] [PubMed] [Google Scholar]
- Rinnerthaler G., Geiger B., Small J. V. Contact formation during fibroblast locomotion: involvement of membrane ruffles and microtubules. J Cell Biol. 1988 Mar;106(3):747–760. doi: 10.1083/jcb.106.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Autocrine activities of basic fibroblast growth factor: regulation of endothelial cell movement, plasminogen activator synthesis, and DNA synthesis. J Cell Biol. 1988 Sep;107(3):1199–1205. doi: 10.1083/jcb.107.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel W., Wollheim C. B. Thyrotropin-releasing hormone increases cytosolic free Ca2+ in clonal pituitary cells (GH3 cells): direct evidence for the mobilization of cellular calcium. J Cell Biol. 1984 Jul;99(1 Pt 1):83–87. doi: 10.1083/jcb.99.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M., Benditt E. P. Clustering of replicating cells in aortic endothelium. Proc Natl Acad Sci U S A. 1976 Feb;73(2):651–653. doi: 10.1073/pnas.73.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Friedman J., Abraham J. A., Fiddes J. C., Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987 Jan 15;325(6101):257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Selden S. C., 3rd, Rabinovitch P. S., Schwartz S. M. Effects of cytoskeletal disrupting agents on replication of bovine endothelium. J Cell Physiol. 1981 Aug;108(2):195–211. doi: 10.1002/jcp.1041080210. [DOI] [PubMed] [Google Scholar]
- Selden S. C., 3rd, Schwartz S. M. Cytochalasin B inhibition of endothelial proliferation at wound edges in vitro. J Cell Biol. 1979 May;81(2):348–354. doi: 10.1083/jcb.81.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholley M. M., Gimbrone M. A., Jr, Cotran R. S. Cellular migration and replication in endothelial regeneration: a study using irradiated endothelial cultures. Lab Invest. 1977 Jan;36(1):18–25. [PubMed] [Google Scholar]
- Shreeniwas R., Ogawa S., Cozzolino F., Torcia G., Braunstein N., Butura C., Brett J., Lieberman H. B., Furie M. B., Joseph-Silverstein J. Macrovascular and microvascular endothelium during long-term hypoxia: alterations in cell growth, monolayer permeability, and cell surface coagulant properties. J Cell Physiol. 1991 Jan;146(1):8–17. doi: 10.1002/jcp.1041460103. [DOI] [PubMed] [Google Scholar]
- Teng M. H., Bartholomew J. C., Bissell M. J. Synergism between anti-microtubule agents and growth stimulants in enhancement of cell cycle traverse. Nature. 1977 Aug 25;268(5622):739–741. doi: 10.1038/268739a0. [DOI] [PubMed] [Google Scholar]
- Ueno H., Gunn M., Dell K., Tseng A., Jr, Williams L. A truncated form of fibroblast growth factor receptor 1 inhibits signal transduction by multiple types of fibroblast growth factor receptor. J Biol Chem. 1992 Jan 25;267(3):1470–1476. [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek A., Haarer B., Field J., Gerst J., Pollard T. D., Brown S., Wigler M. Evidence for a functional link between profilin and CAP in the yeast S. cerevisiae. Cell. 1991 Aug 9;66(3):497–505. doi: 10.1016/0092-8674(81)90013-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Kardami E., Nagy J. I. Basic fibroblast growth factor in rat brain: localization to glial gap junctions correlates with connexin43 distribution. Brain Res. 1991 Jul 19;554(1-2):336–343. doi: 10.1016/0006-8993(91)90213-f. [DOI] [PubMed] [Google Scholar]