Abstract
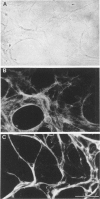
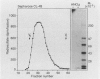
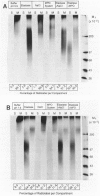
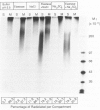
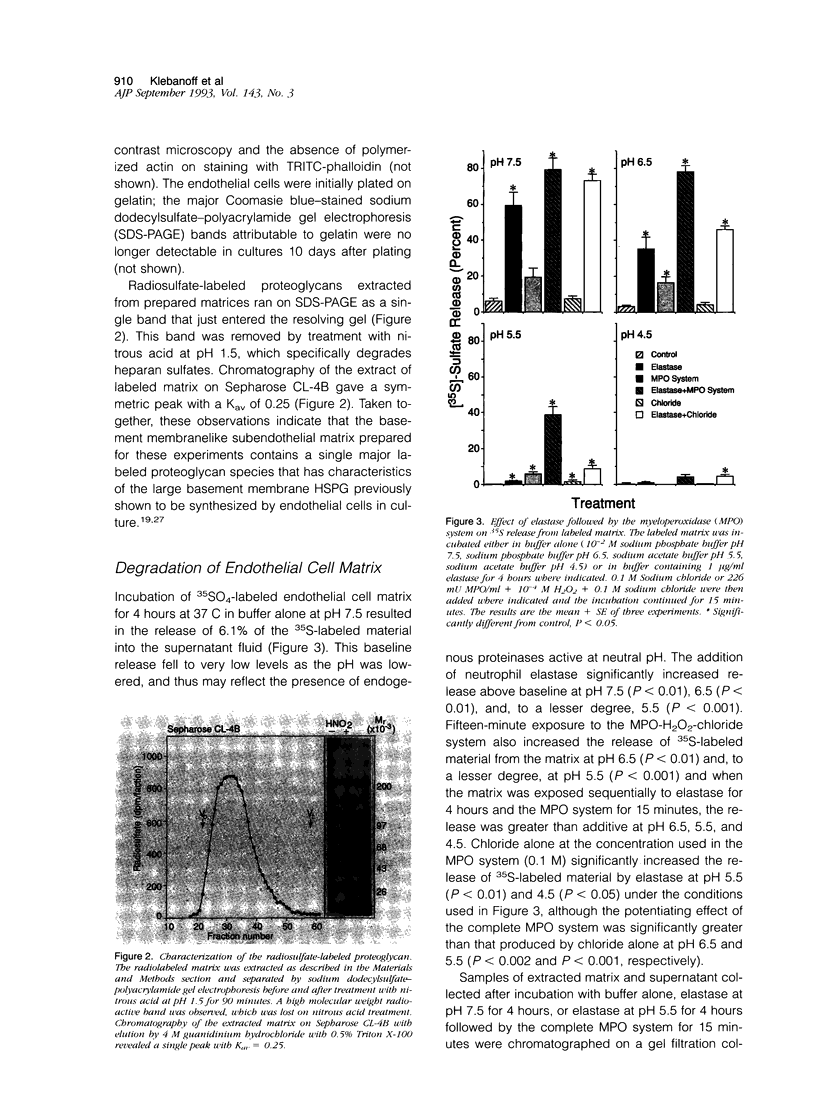
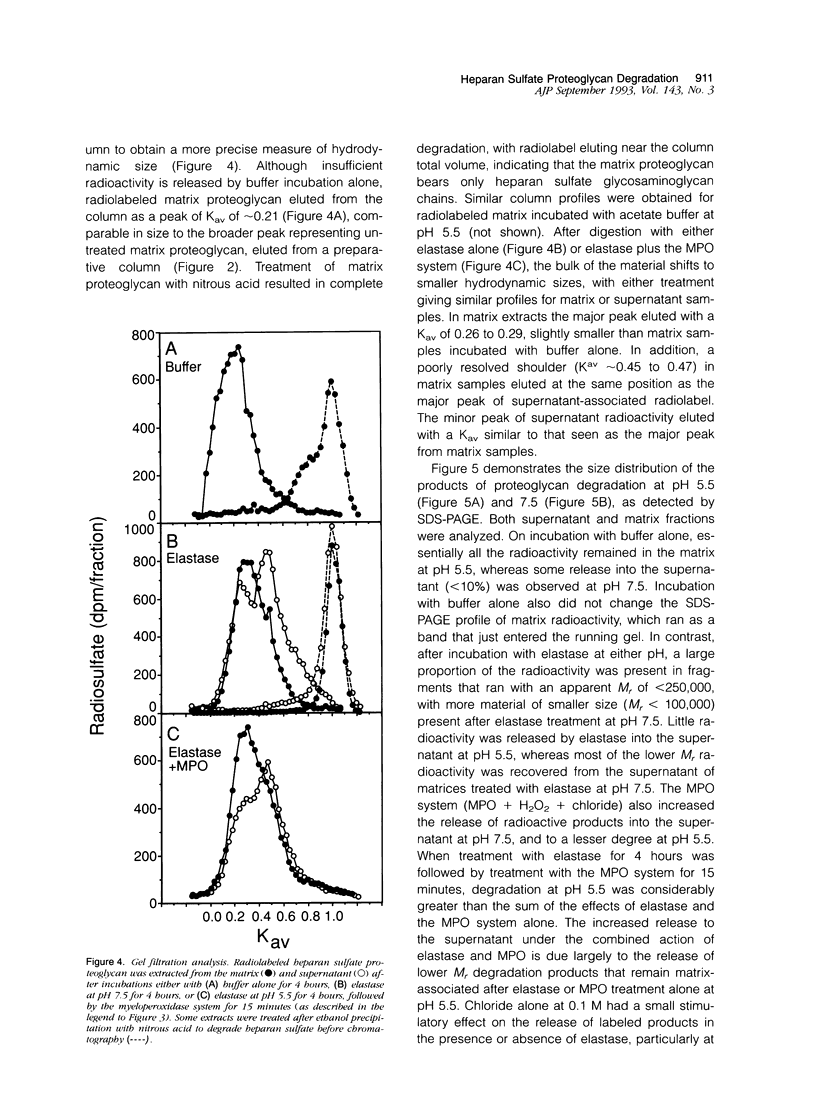
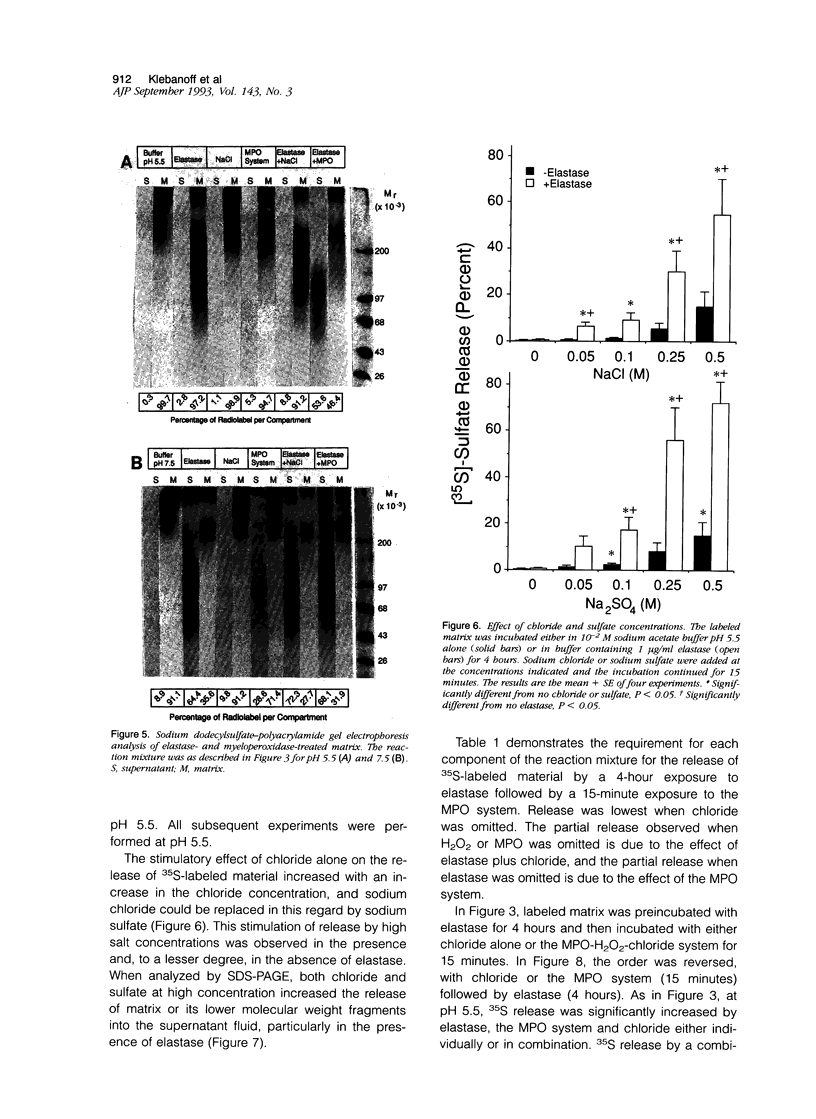
The degradation of the heparan sulfate proteoglycans of subendothelial matrix by neutrophil elastase and the myeloperoxidase-H2O2-chloride system added separately, sequentially, or together at pH 4.5 to 7.5 was determined by the release of lower molecular weight 35S-labeled material. Elastase alone and the myeloperoxidase system alone caused degradation, and when 4-hour exposure to elastase was followed by 15 minutes of exposure to the myeloperoxidase system, the effect was greater than additive. A greater than additive effect was not observed when elastase followed the myeloperoxidase system or the two were added together. Chloride (or sulfate) alone increased the release of 35S-labeled material from elastase-treated matrix, although the effect of 0.1 M chloride was not as great as that observed when an equivalent concentration of chloride was combined with myeloperoxidase and H2O2. The release of these systems at sites of adherence of neutrophils to glomerular basement membrane may contribute to neutrophil-associated proteinuria.
Full text
PDF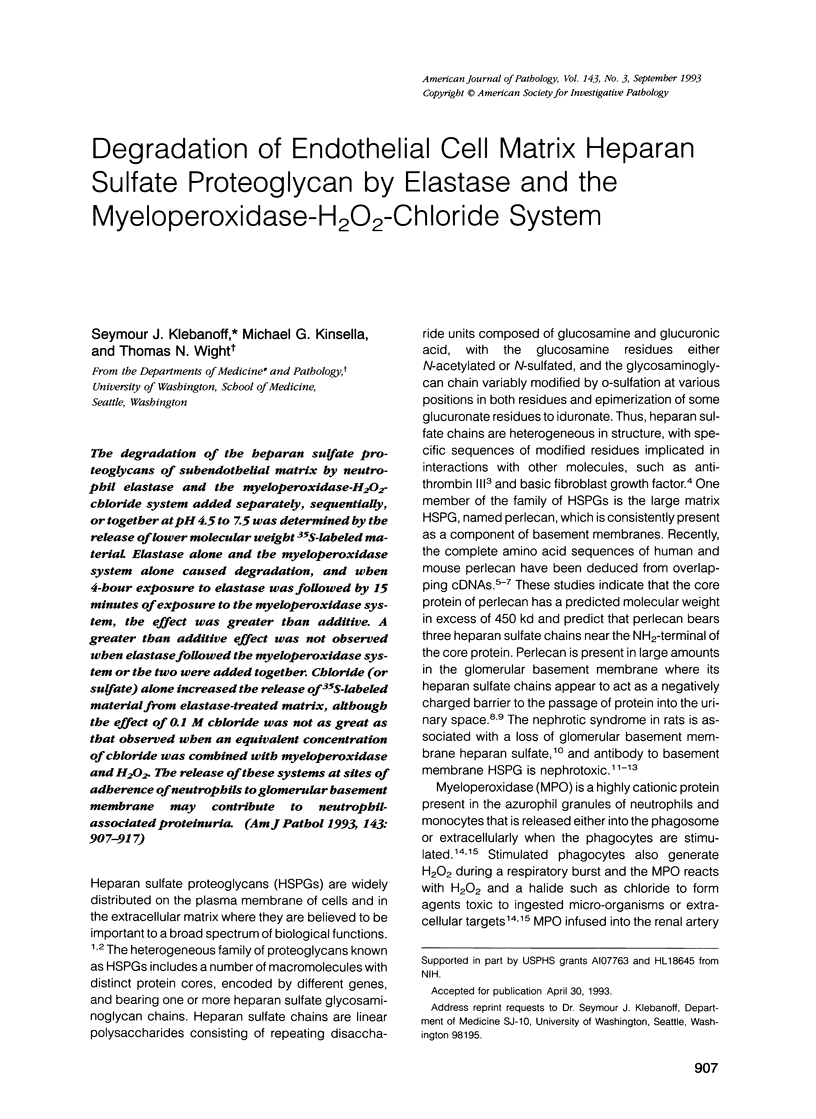






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrass C. K., Cohen A. H. Characterization of renal injury initiated by immunization of rats with heparan sulfate. Am J Pathol. 1988 Jan;130(1):103–111. [PMC free article] [PubMed] [Google Scholar]
- Baici A., Bradamante P. Interaction between human leukocyte elastase and chondroitin sulfate. Chem Biol Interact. 1984 Sep 1;51(1):1–11. doi: 10.1016/0009-2797(84)90015-2. [DOI] [PubMed] [Google Scholar]
- Baici A., Salgam P., Fehr K., Böni A. Inhibition of human elastase from polymorphonuclear leucocytes by a glycosaminoglycan polysulfate (Arteparon). Biochem Pharmacol. 1980 Jun 15;29(12):1723–1727. doi: 10.1016/0006-2952(80)90131-8. [DOI] [PubMed] [Google Scholar]
- Baker M. S., Green S. P., Lowther D. A. Changes in the viscosity of hyaluronic acid after exposure to a myeloperoxidase-derived oxidant. Arthritis Rheum. 1989 Apr;32(4):461–467. doi: 10.1002/anr.1780320416. [DOI] [PubMed] [Google Scholar]
- Bar-Ner M., Eldor A., Wasserman L., Matzner Y., Cohen I. R., Fuks Z., Vlodavsky I. Inhibition of heparanase-mediated degradation of extracellular matrix heparan sulfate by non-anticoagulant heparin species. Blood. 1987 Aug;70(2):551–557. [PubMed] [Google Scholar]
- Gallagher J. T., Lyon M., Steward W. P. Structure and function of heparan sulphate proteoglycans. Biochem J. 1986 Jun 1;236(2):313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. P., Baker M. S., Lowther D. A. Depolymerization of synovial fluid hyaluronic acid (HA) by the complete myeloperoxidase (MPO) system may involve the formation of a HA-MPO ionic complex. J Rheumatol. 1990 Dec;17(12):1670–1675. [PubMed] [Google Scholar]
- Janusz M. J., Doherty N. S. Degradation of cartilage matrix proteoglycan by human neutrophils involves both elastase and cathepsin G. J Immunol. 1991 Jun 1;146(11):3922–3928. [PubMed] [Google Scholar]
- Johnson R. J., Couser W. G., Alpers C. E., Vissers M., Schulze M., Klebanoff S. J. The human neutrophil serine proteinases, elastase and cathepsin G, can mediate glomerular injury in vivo. J Exp Med. 1988 Sep 1;168(3):1169–1174. doi: 10.1084/jem.168.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Couser W. G., Chi E. Y., Adler S., Klebanoff S. J. New mechanism for glomerular injury. Myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest. 1987 May;79(5):1379–1387. doi: 10.1172/JCI112965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Guggenheim S. J., Klebanoff S. J., Ochi R. F., Wass A., Baker P., Schulze M., Couser W. G. Morphologic correlates of glomerular oxidant injury induced by the myeloperoxidase-hydrogen peroxide-halide system of the neutrophil. Lab Invest. 1988 Mar;58(3):294–301. [PubMed] [Google Scholar]
- Kallunki P., Tryggvason K. Human basement membrane heparan sulfate proteoglycan core protein: a 467-kD protein containing multiple domains resembling elements of the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J Cell Biol. 1992 Jan;116(2):559–571. doi: 10.1083/jcb.116.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Linker A., Farquhar M. G. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol. 1980 Aug;86(2):688–693. doi: 10.1083/jcb.86.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katrantzis M., Baker M. S., Handley C. J., Lowther D. A. The oxidant hypochlorite (OCl-), a product of the myeloperoxidase system, degrades articular cartilage proteoglycan aggregate. Free Radic Biol Med. 1991;10(2):101–109. doi: 10.1016/0891-5849(91)90003-l. [DOI] [PubMed] [Google Scholar]
- Key N. S., Platt J. L., Vercellotti G. M. Vascular endothelial cell proteoglycans are susceptible to cleavage by neutrophils. Arterioscler Thromb. 1992 Jul;12(7):836–842. doi: 10.1161/01.atv.12.7.836. [DOI] [PubMed] [Google Scholar]
- Kinsella M. G., Wight T. N. Modulation of sulfated proteoglycan synthesis by bovine aortic endothelial cells during migration. J Cell Biol. 1986 Mar;102(3):679–687. doi: 10.1083/jcb.102.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella M. G., Wight T. N. Structural characterization of heparan sulfate proteoglycan subclasses isolated from bovine aortic endothelial cell cultures. Biochemistry. 1988 Mar 22;27(6):2136–2144. doi: 10.1021/bi00406a048. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Waltersdorph A. M., Rosen H. Antimicrobial activity of myeloperoxidase. Methods Enzymol. 1984;105:399–403. doi: 10.1016/s0076-6879(84)05055-2. [DOI] [PubMed] [Google Scholar]
- Kowanko I. C., Bates E. J., Ferrante A. Mechanisms of human neutrophil-mediated cartilage damage in vitro: the role of lysosomal enzymes, hydrogen peroxide and hypochlorous acid. Immunol Cell Biol. 1989 Oct;67(Pt 5):321–329. doi: 10.1038/icb.1989.47. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Vogel K. G., Nicolson G. L. Solubilization and degradation of subendothelial matrix glycoproteins and proteoglycans by metastatic tumor cells. J Biol Chem. 1982 Mar 10;257(5):2678–2686. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Jansson L., Hallén A. Biosynthesis of heparin. II. Formation of sulfamino groups. J Biol Chem. 1973 Oct 25;248(20):7234–7241. [PubMed] [Google Scholar]
- Lindahl U., Thunberg L., Bäckström G., Riesenfeld J., Nordling K., Björk I. Extension and structural variability of the antithrombin-binding sequence in heparin. J Biol Chem. 1984 Oct 25;259(20):12368–12376. [PubMed] [Google Scholar]
- Makino H., Gibbons J. T., Reddy M. K., Kanwar Y. S. Nephritogenicity of antibodies to proteoglycans of the glomerular basement membrane--I. J Clin Invest. 1986 Jan;77(1):142–156. doi: 10.1172/JCI112269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzner Y., Bar-Ner M., Yahalom J., Ishai-Michaeli R., Fuks Z., Vlodavsky I. Degradation of heparan sulfate in the subendothelial extracellular matrix by a readily released heparanase from human neutrophils. Possible role in invasion through basement membranes. J Clin Invest. 1985 Oct;76(4):1306–1313. doi: 10.1172/JCI112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzner Y., Vlodavsky I., Bar-Ner M., Ishai-Michaeli R., Tauber A. I. Subcellular localization of heparanase in human neutrophils. J Leukoc Biol. 1992 Jun;51(6):519–524. doi: 10.1002/jlb.51.6.519. [DOI] [PubMed] [Google Scholar]
- McGowan S. E. Mechanisms of extracellular matrix proteoglycan degradation by human neutrophils. Am J Respir Cell Mol Biol. 1990 Mar;2(3):271–279. doi: 10.1165/ajrcmb/2.3.271. [DOI] [PubMed] [Google Scholar]
- McGowan S. E., Murray J. J. Direct effects of neutrophil oxidants on elastase-induced extracellular matrix proteolysis. Am Rev Respir Dis. 1987 Jun;135(6):1286–1293. doi: 10.1164/arrd.1987.135.6.1286. [DOI] [PubMed] [Google Scholar]
- McGowan S. E., Thompson R. J. Extracellular matrix proteoglycan degradation by human alveolar macrophages and neutrophils. J Appl Physiol (1985) 1989 Jan;66(1):400–409. doi: 10.1152/jappl.1989.66.1.400. [DOI] [PubMed] [Google Scholar]
- Miettinen A., Stow J. L., Mentone S., Farquhar M. G. Antibodies to basement membrane heparan sulfate proteoglycans bind to the laminae rarae of the glomerular basement membrane (GBM) and induce subepithelial GBM thickening. J Exp Med. 1986 May 1;163(5):1064–1084. doi: 10.1084/jem.163.5.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch A. D., Dodge G. R., Cohen I., Tuan R. S., Iozzo R. V. Primary structure of the human heparan sulfate proteoglycan from basement membrane (HSPG2/perlecan). A chimeric molecule with multiple domains homologous to the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J Biol Chem. 1992 Apr 25;267(12):8544–8557. [PubMed] [Google Scholar]
- Noonan D. M., Fulle A., Valente P., Cai S., Horigan E., Sasaki M., Yamada Y., Hassell J. R. The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J Biol Chem. 1991 Dec 5;266(34):22939–22947. [PubMed] [Google Scholar]
- Rakita R. M., Michel B. R., Rosen H. Differential inactivation of Escherichia coli membrane dehydrogenases by a myeloperoxidase-mediated antimicrobial system. Biochemistry. 1990 Jan 30;29(4):1075–1080. doi: 10.1021/bi00456a033. [DOI] [PubMed] [Google Scholar]
- Rosenzweig L. J., Kanwar Y. S. Removal of sulfated (heparan sulfate) or nonsulfated (hyaluronic acid) glycosaminoglycans results in increased permeability of the glomerular basement membrane to 125I-bovine serum albumin. Lab Invest. 1982 Aug;47(2):177–184. [PubMed] [Google Scholar]
- Saku T., Furthmayr H. Characterization of the major heparan sulfate proteoglycan secreted by bovine aortic endothelial cells in culture. Homology to the large molecular weight molecule of basement membranes. J Biol Chem. 1989 Feb 25;264(6):3514–3523. [PubMed] [Google Scholar]
- Turnbull J. E., Fernig D. G., Ke Y., Wilkinson M. C., Gallagher J. T. Identification of the basic fibroblast growth factor binding sequence in fibroblast heparan sulfate. J Biol Chem. 1992 May 25;267(15):10337–10341. [PubMed] [Google Scholar]
- Vissers M. C., Day W. A., Winterbourn C. C. Neutrophils adherent to a nonphagocytosable surface (glomerular basement membrane) produce oxidants only at the site of attachment. Blood. 1985 Jul;66(1):161–166. [PubMed] [Google Scholar]
- Vissers M. C., Winterbourn C. C., Hunt J. S. Degradation of glomerular basement membrane by human neutrophils in vitro. Biochim Biophys Acta. 1984 Jun 19;804(2):154–160. doi: 10.1016/0167-4889(84)90144-7. [DOI] [PubMed] [Google Scholar]
- Wasteson A., Uthne K., Westermark B. A novel assay for the biosynthesis of sulphated polysaccharide and its application to studies on the effects of somatomedin on cultured cells. Biochem J. 1973 Dec;136(4):1069–1074. doi: 10.1042/bj1361069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Curnutte J. T., Regiani S. Neutrophil-mediated solubilization of the subendothelial matrix: oxidative and nonoxidative mechanisms of proteolysis used by normal and chronic granulomatous disease phagocytes. J Immunol. 1986 Jan;136(2):636–641. [PubMed] [Google Scholar]
- Weiss S. J., Regiani S. Neutrophils degrade subendothelial matrices in the presence of alpha-1-proteinase inhibitor. Cooperative use of lysosomal proteinases and oxygen metabolites. J Clin Invest. 1984 May;73(5):1297–1303. doi: 10.1172/JCI111332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Cell surface heparan sulfate proteoglycans. J Biol Chem. 1992 May 15;267(14):9451–9454. [PubMed] [Google Scholar]