Abstract
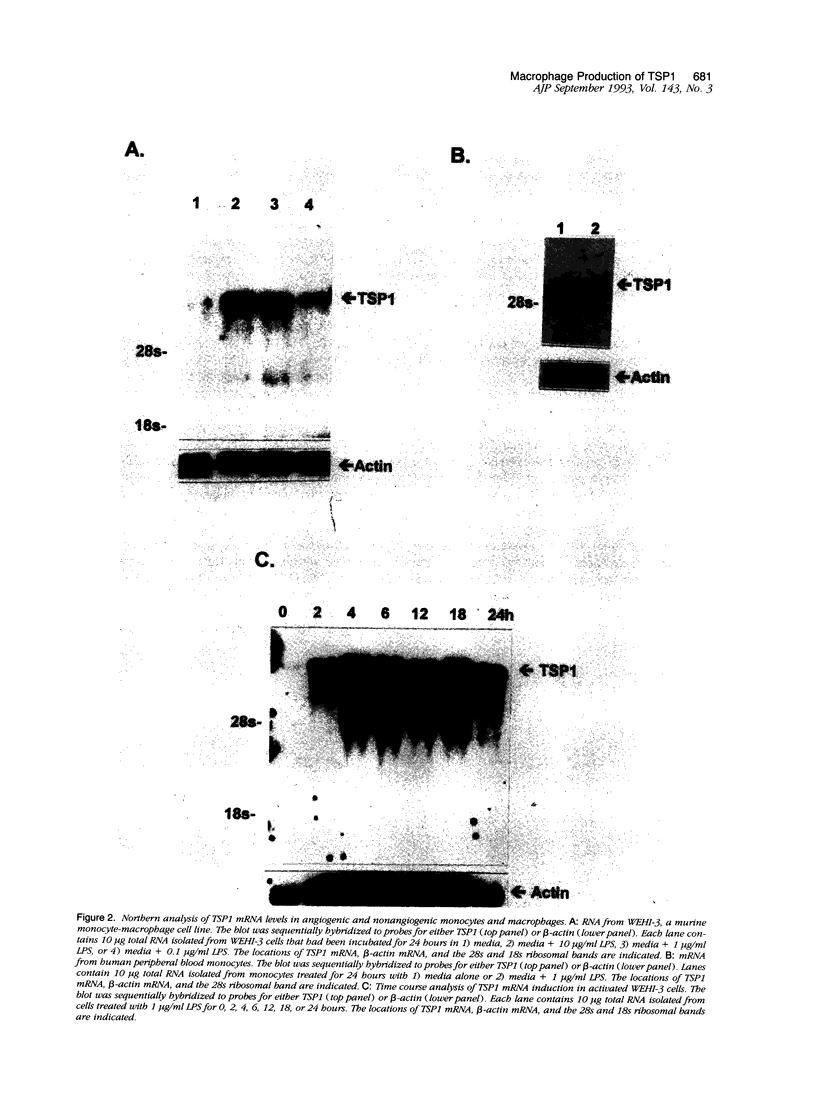
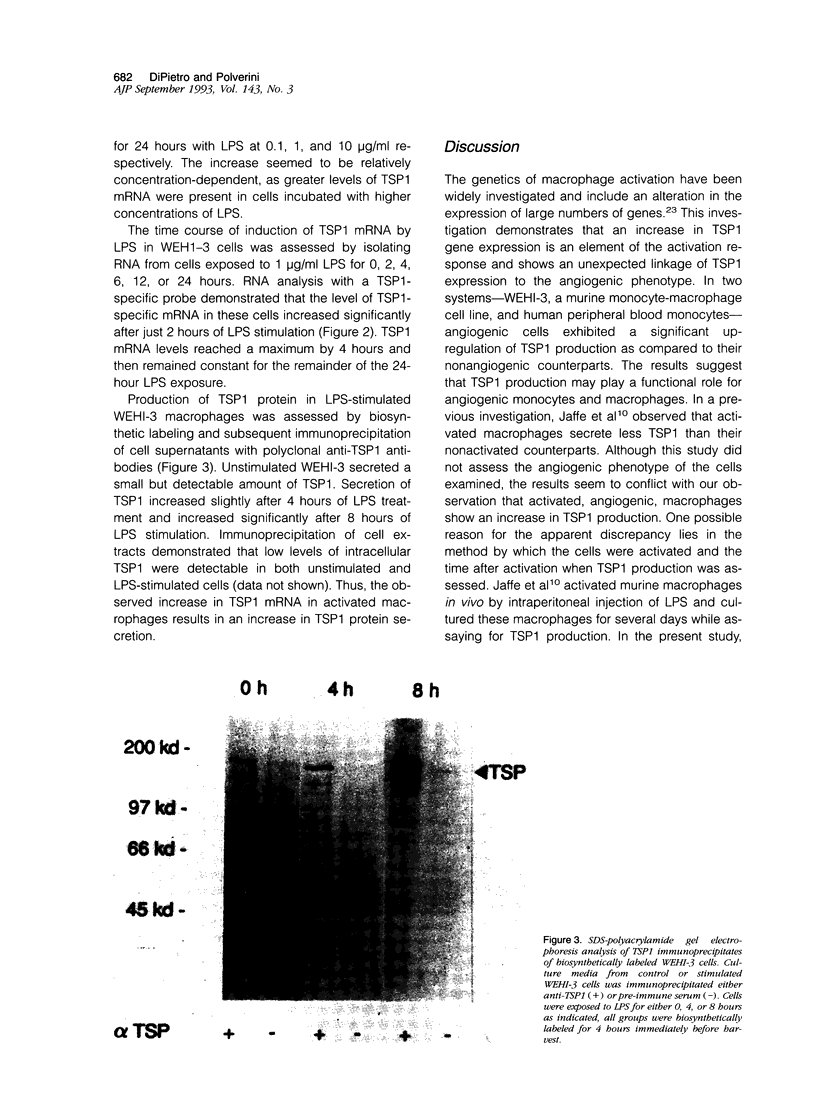
Previous investigations have shown that macrophages play a pivotal role in the induction of angiogenesis in both physiological and pathological settings. This investigation examines the relative production of the angiogenic modulator thrombospondin-1 (TSP1) by activated and nonactivated monocytes and macrophages. TSP1, a multifunctional extracellular matrix molecule, has been reported recently to inhibit angiogenesis both in vitro and in vivo. To examine the relationship between the level of TSP1 production by macrophages and expression of the angiogenic phenotype, murine monocytelike cells (WEHI-3) and human peripheral blood monocytes were each activated in vitro and examined for TSP1 production and angiogenic activity in rat corneal bioassay. Nonangiogenic monocytes produced low levels of TSP1 messenger RNA. Surprisingly, activated, potently angiogenic monocytes and macrophages exhibited as much as a sixfold increase in steady state TSP1 messenger RNA over unstimulated levels. Biosynthetic labeling studies demonstrated that TSP1 protein secretion increased in conjunction with increased TSP1 messenger RNA levels in angiogenic macrophages. The results demonstrate that activated monocytes and macrophages actively produce the angiogenic modulator TSP1 and suggest that TSP1 production may be a component of the angiogenic phenotype. In addition, the data suggest that the ability of macrophages to mediate angiogenesis results from a complex interplay of positive and negative regulators.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Koerner T. J. Gene regulation in macrophage development and activation. Year Immunol. 1989;4:159–180. [PubMed] [Google Scholar]
- Bagavandoss P., Wilks J. W. Specific inhibition of endothelial cell proliferation by thrombospondin. Biochem Biophys Res Commun. 1990 Jul 31;170(2):867–872. doi: 10.1016/0006-291x(90)92171-u. [DOI] [PubMed] [Google Scholar]
- Besner G. E., Klagsbrun M. Macrophages secrete a heparin-binding inhibitor of endothelial cell growth. Microvasc Res. 1991 Sep;42(2):187–197. doi: 10.1016/0026-2862(91)90086-q. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Frazier W. A. Thrombospondin: a modular adhesive glycoprotein of platelets and nucleated cells. J Cell Biol. 1987 Aug;105(2):625–632. doi: 10.1083/jcb.105.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good D. J., Polverini P. J., Rastinejad F., Le Beau M. M., Lemons R. S., Frazier W. A., Bouck N. P. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy S. W., Frazier B. A., Kim D. D., Deckwerth T. L., Baumgartel D. M., Rotwein P., Frazier W. A. Complete thrombospondin mRNA sequence includes potential regulatory sites in the 3' untranslated region. J Cell Biol. 1989 Feb;108(2):729–736. doi: 10.1083/jcb.108.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg P. J., Stenflo J., Mosher D. F. Thrombospondin is a slow tight-binding inhibitor of plasmin. Biochemistry. 1992 Jan 14;31(1):265–269. doi: 10.1021/bi00116a036. [DOI] [PubMed] [Google Scholar]
- Huber A. R., Ellis S., Johnson K. J., Dixit V. M., Varani J. Monocyte diapedesis through an in vitro vessel wall construct: inhibition with monoclonal antibodies to thrombospondin. J Leukoc Biol. 1992 Nov;52(5):524–528. doi: 10.1002/jlb.52.5.524. [DOI] [PubMed] [Google Scholar]
- Hunt T. K., Knighton D. R., Thakral K. K., Goodson W. H., 3rd, Andrews W. S. Studies on inflammation and wound healing: angiogenesis and collagen synthesis stimulated in vivo by resident and activated wound macrophages. Surgery. 1984 Jul;96(1):48–54. [PubMed] [Google Scholar]
- Iruela-Arispe M. L., Bornstein P., Sage H. Thrombospondin exerts an antiangiogenic effect on cord formation by endothelial cells in vitro. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5026–5030. doi: 10.1073/pnas.88.11.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Ruggiero J. T., Falcone D. J. Monocytes and macrophages synthesize and secrete thrombospondin. Blood. 1985 Jan;65(1):79–84. [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Leibovich S. J. Induction of neovascularization by activated human monocytes. J Leukoc Biol. 1986 Feb;39(2):233–238. doi: 10.1002/jlb.39.2.233. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Leibovich S. J. Stimulation of neovascularization by human rheumatoid synovial tissue macrophages. Arthritis Rheum. 1986 Apr;29(4):471–479. doi: 10.1002/art.1780290403. [DOI] [PubMed] [Google Scholar]
- Lawler J. The structural and functional properties of thrombospondin. Blood. 1986 May;67(5):1197–1209. [PubMed] [Google Scholar]
- Mansfield P. J., Boxer L. A., Suchard S. J. Thrombospondin stimulates motility of human neutrophils. J Cell Biol. 1990 Dec;111(6 Pt 2):3077–3086. doi: 10.1083/jcb.111.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard E., Flodgaard H. A neutrophil-derived proteolytic inactive elastase homologue (hHBP) mediates reversible contraction of fibroblasts and endothelial cell monolayers and stimulates monocyte survival and thrombospondin secretion. J Leukoc Biol. 1992 Apr;51(4):316–323. doi: 10.1002/jlb.51.4.316. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Leibovich S. J. Induction of neovascularization in vivo and endothelial proliferation in vitro by tumor-associated macrophages. Lab Invest. 1984 Dec;51(6):635–642. [PubMed] [Google Scholar]
- Porras-Reyes B. H., Blair H. C., Jeffrey J. J., Mustoe T. A. Collagenase production at the border of granulation tissue in a healing wound: macrophage and mesenchymal collagenase production in vivo. Connect Tissue Res. 1991;27(1):63–71. doi: 10.3109/03008209109006995. [DOI] [PubMed] [Google Scholar]
- Rastinejad F., Polverini P. J., Bouck N. P. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell. 1989 Feb 10;56(3):345–355. doi: 10.1016/0092-8674(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Savill J., Hogg N., Ren Y., Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992 Oct;90(4):1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein R. L., Nachman R. L. Thrombospondin binds to monocytes-macrophages and mediates platelet-monocyte adhesion. J Clin Invest. 1987 Mar;79(3):867–874. doi: 10.1172/JCI112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderkötter C., Goebeler M., Schulze-Osthoff K., Bhardwaj R., Sorg C. Macrophage-derived angiogenesis factors. Pharmacol Ther. 1991;51(2):195–216. doi: 10.1016/0163-7258(91)90077-y. [DOI] [PubMed] [Google Scholar]
- Taraboletti G., Roberts D., Liotta L. A., Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol. 1990 Aug;111(2):765–772. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral K. K., Goodson W. H., 3rd, Hunt T. K. Stimulation of wound blood vessel growth by wound macrophages. J Surg Res. 1979 Apr;26(4):430–436. doi: 10.1016/0022-4804(79)90031-3. [DOI] [PubMed] [Google Scholar]
- Varani J., Stoolman L., Wang T., Schuger L., Flippen C., Dame M., Johnson K. J., Todd R. F., 3rd, Ryan U. S., Ward P. A. Thrombospondin production and thrombospondin-mediated adhesion in U937 cells. Exp Cell Res. 1991 Jul;195(1):177–182. doi: 10.1016/0014-4827(91)90514-u. [DOI] [PubMed] [Google Scholar]
- Vissers M. C., Jester S. A., Fantone J. C. Rapid purification of human peripheral blood monocytes by centrifugation through Ficoll-Hypaque and Sepracell-MN. J Immunol Methods. 1988 Jun 13;110(2):203–207. doi: 10.1016/0022-1759(88)90104-4. [DOI] [PubMed] [Google Scholar]