Abstract
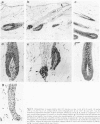
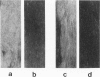
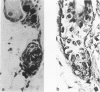
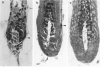
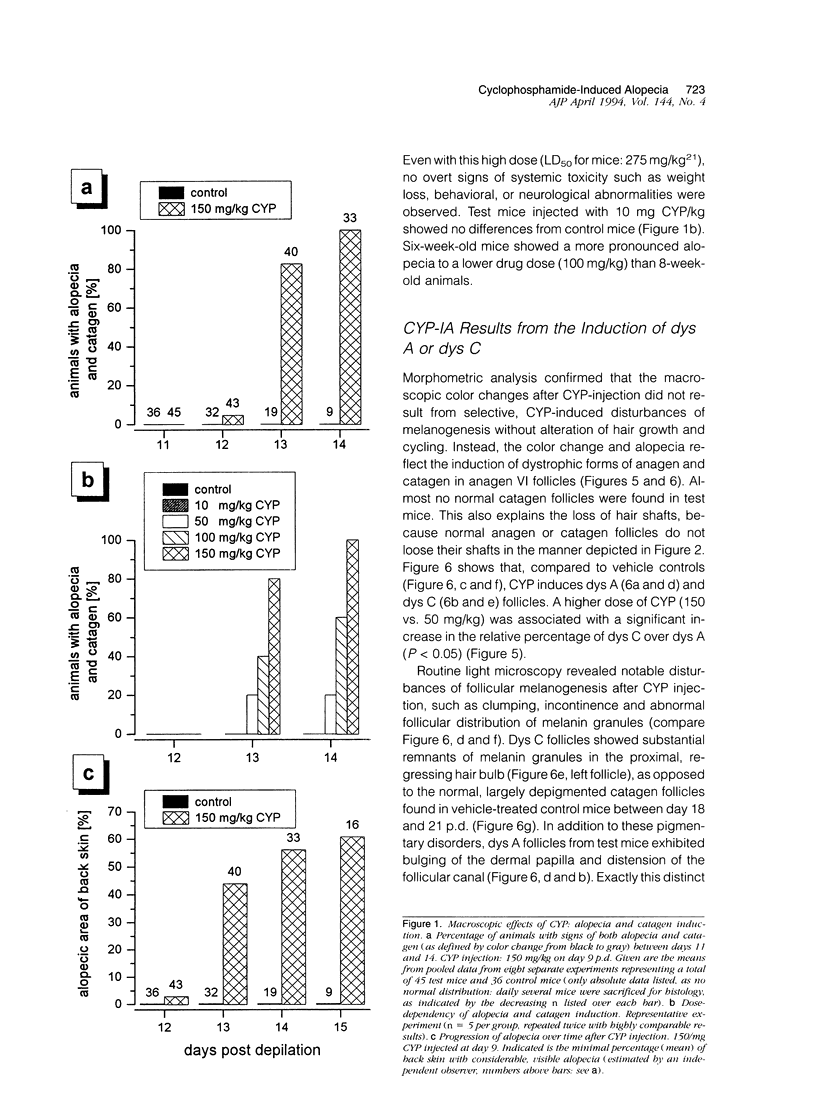
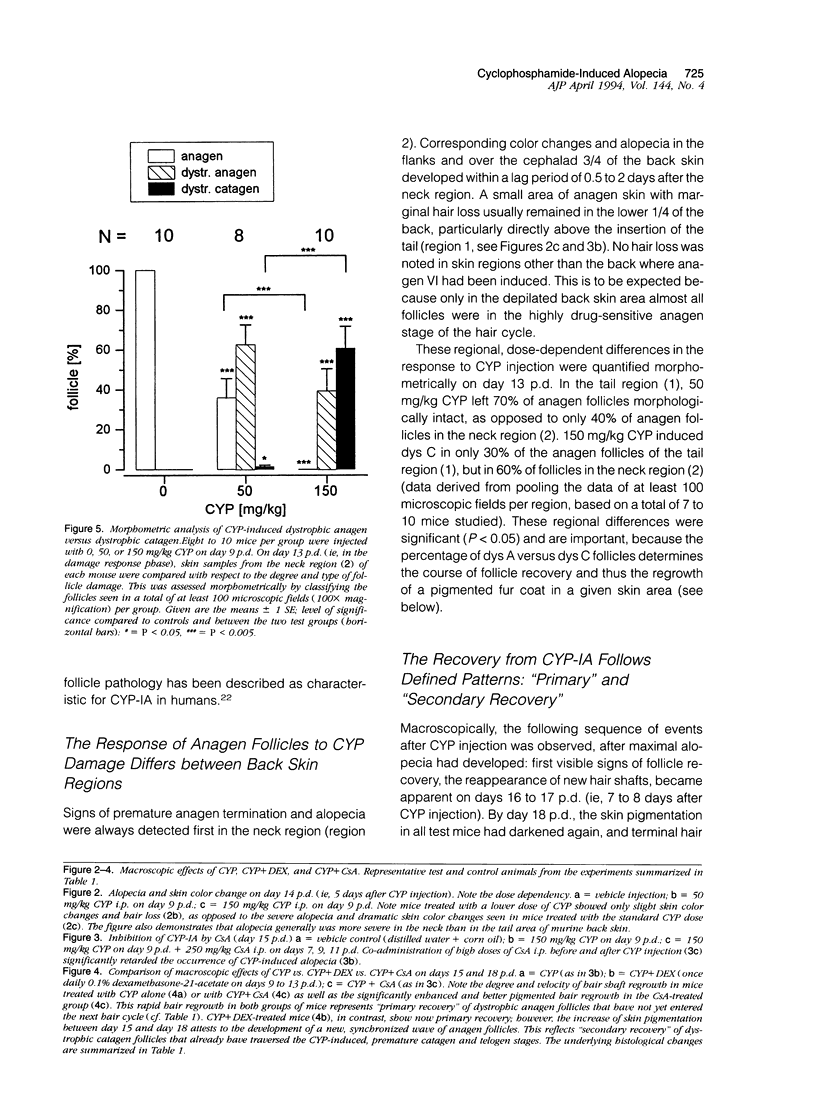
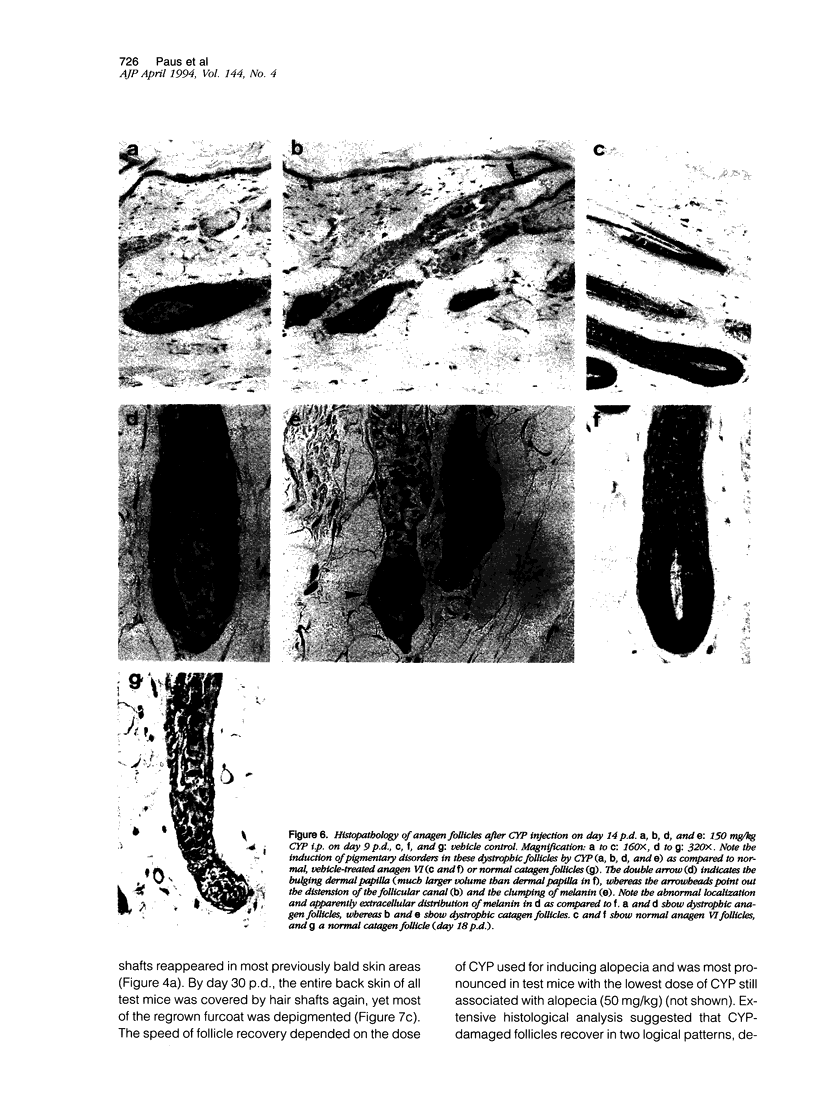
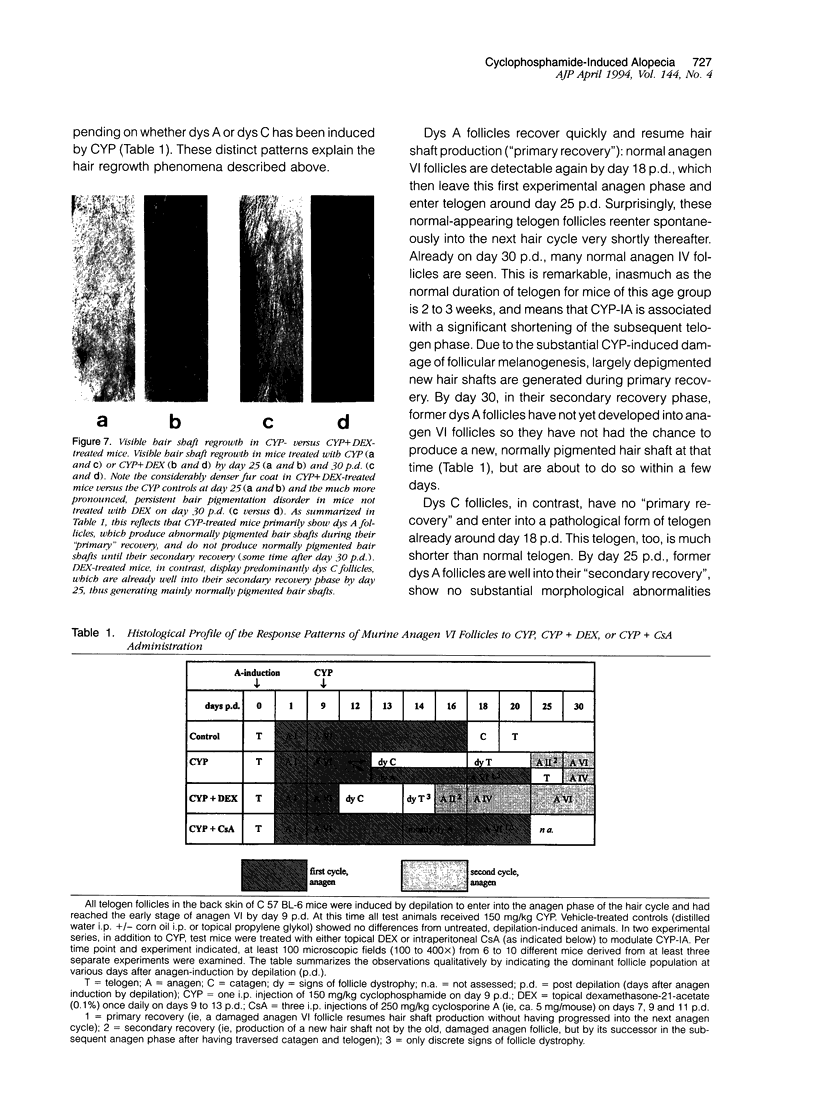
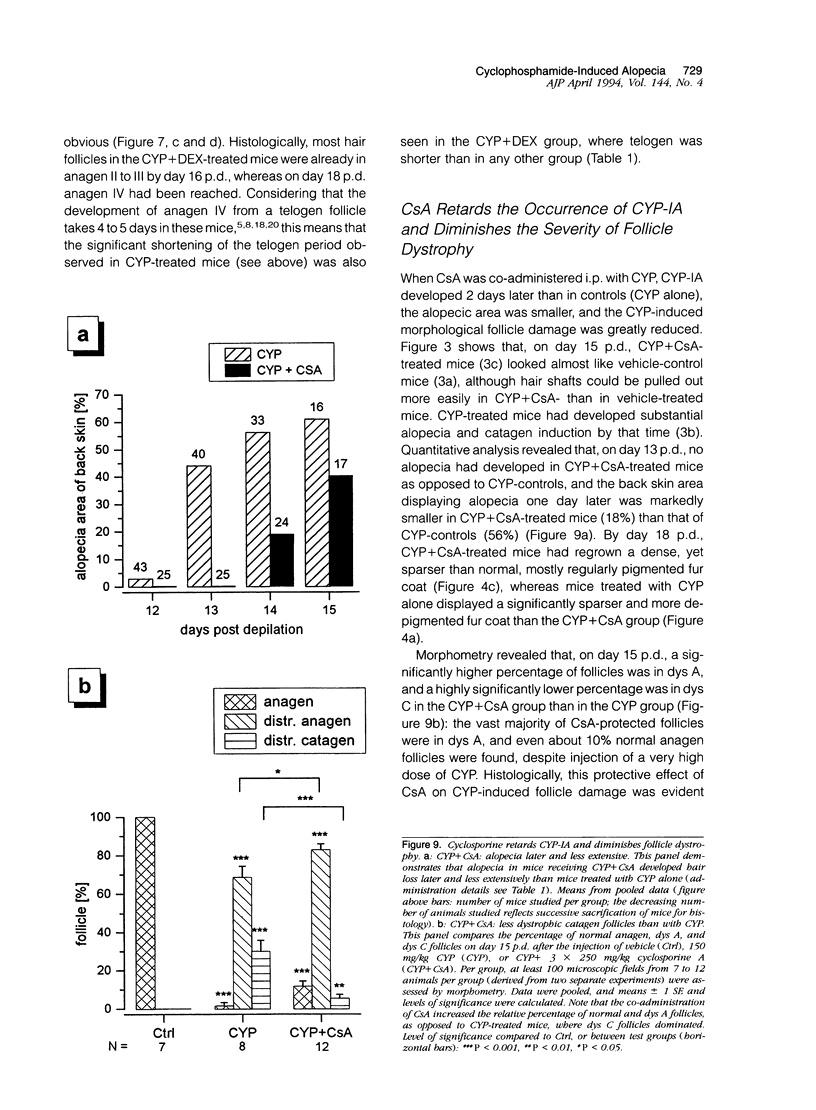
We introduce cyclophosphamide-induced alopecia (CYP-IA) in C57BL-6 mice as a clinically relevant model for studying the biology of chemotherapy-induced alopecia and for developing anti-alopecia drugs. One injection of CYP to mice with all back skin follicles in anagen VI induces severe alopecia that strikingly reproduces the follicle response, recovery, and histopathology seen in human CYP-IA. CYP dose-dependently induces abnormal follicular melanogenesis and dystrophic anagen or, in more severely damaged follicles, dystrophic catagen. Both dystrophy forms are followed by an extremely shortened telogen phase, but differ in the associated hair loss and in recovery patterns, which determines hair regrowth. This follicular response to CYP can be manipulated pharmacologically: systemic cyclosporine A shifts it toward a mild form of dystrophic anagen, thus retarding CYP-IA and prolonging "primary recovery". Topical dexamethasone, in contrast, forces follicles into dystrophic catagen, which augments CYP-IA, but accelerates the regrowth of normally pigmented hair ("secondary recovery").
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHASE H. B. Growth of the hair. Physiol Rev. 1954 Jan;34(1):113–126. doi: 10.1152/physrev.1954.34.1.113. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C., Fadok V. A., Sellins K. S. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G., Sun T. T., Lavker R. M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990 Jun 29;61(7):1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Goldberg M. T., Tackaberry L. E., Hardy M. H., Noseworthy J. H. Nuclear aberrations in hair follicle cells of patients receiving cyclophosphamide. A possible in vivo assay for human exposure to genotoxic agents. Arch Toxicol. 1990;64(2):116–121. doi: 10.1007/BF01974396. [DOI] [PubMed] [Google Scholar]
- Hardy M. H. The secret life of the hair follicle. Trends Genet. 1992 Feb;8(2):55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- Headington J. T. Telogen effluvium. New concepts and review. Arch Dermatol. 1993 Mar;129(3):356–363. doi: 10.1001/archderm.129.3.356. [DOI] [PubMed] [Google Scholar]
- Hussein A. M. Interleukin 1 protects against 1-beta-D-arabinofuranosylcytosine-induced alopecia in the newborn rat animal model. Cancer Res. 1991 Jun 15;51(12):3329–3330. [PubMed] [Google Scholar]
- Hussein A. M., Jimenez J. J., McCall C. A., Yunis A. A. Protection from chemotherapy-induced alopecia in a rat model. Science. 1990 Sep 28;249(4976):1564–1566. doi: 10.1126/science.2218498. [DOI] [PubMed] [Google Scholar]
- Jimenez J. J., Wong G. H., Yunis A. A. Interleukin 1 protects from cytosine arabinoside-induced alopecia in the rat model. FASEB J. 1991 Jul;5(10):2456–2458. doi: 10.1096/fasebj.5.10.2065892. [DOI] [PubMed] [Google Scholar]
- Jimenez J. J., Yunis A. A. Protection from chemotherapy-induced alopecia by 1,25-dihydroxyvitamin D3. Cancer Res. 1992 Sep 15;52(18):5123–5125. [PubMed] [Google Scholar]
- Kostanecki W., Kwiatkowska E., Zborzil J. Die Haarmelanogenese bei der Endoxan-Alopecie und deren Beeinflussung durch Corticosteroide. Arch Klin Exp Dermatol. 1966 Sep 14;226(1):13–20. [PubMed] [Google Scholar]
- Li L., Margolis L. B., Paus R., Hoffman R. M. Hair shaft elongation, follicle growth, and spontaneous regression in long-term, gelatin sponge-supported histoculture of human scalp skin. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8764–8768. doi: 10.1073/pnas.89.18.8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Paus R., Slominski A., Hoffman R. M. Skin histoculture assay for studying the hair cycle. In Vitro Cell Dev Biol. 1992 Nov-Dec;28A(11-12):695–698. doi: 10.1007/BF02631052. [DOI] [PubMed] [Google Scholar]
- Paus R., Czarnetzki B. M. Neue Perspektiven in der Haarforschung: auf der Suche nach der "biologischen Uhr" des Haarzyklus. Hautarzt. 1992 May;43(5):264–271. [PubMed] [Google Scholar]
- Paus R., Rosenbach T., Haas N., Czarnetzki B. M. Patterns of cell death: the significance of apoptosis for dermatology. Exp Dermatol. 1993 Feb;2(1):3–11. doi: 10.1111/j.1600-0625.1993.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Paus R., Stenn K. S., Link R. E. Telogen skin contains an inhibitor of hair growth. Br J Dermatol. 1990 Jun;122(6):777–784. doi: 10.1111/j.1365-2133.1990.tb06266.x. [DOI] [PubMed] [Google Scholar]
- Paus R., Stenn K. S., Link R. E. The induction of anagen hair growth in telogen mouse skin by cyclosporine A administration. Lab Invest. 1989 Mar;60(3):365–369. [PubMed] [Google Scholar]
- STRAILE W. E., CHASE H. B., ARSENAULT C. Growth and differentiation of hair follicles between periods of activity and quiescence. J Exp Zool. 1961 Dec;148:205–221. doi: 10.1002/jez.1401480304. [DOI] [PubMed] [Google Scholar]
- Slominski A., Paus R., Costantino R. Differential expression and activity of melanogenesis-related proteins during induced hair growth in mice. J Invest Dermatol. 1991 Feb;96(2):172–179. doi: 10.1111/1523-1747.ep12460956. [DOI] [PubMed] [Google Scholar]
- Slominski A., Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993 Jul;101(1 Suppl):90S–97S. doi: 10.1111/1523-1747.ep12362991. [DOI] [PubMed] [Google Scholar]
- Slominski A., Paus R., Schadendorf D. Melanocytes as "sensory" and regulatory cells in the epidermis. J Theor Biol. 1993 Sep 7;164(1):103–120. doi: 10.1006/jtbi.1993.1142. [DOI] [PubMed] [Google Scholar]
- Stenn K. S., Paus R., Dutton T., Sarba B. Glucocorticoid effect on hair growth initiation: a reconsideration. Skin Pharmacol. 1993;6(2):125–134. doi: 10.1159/000211097. [DOI] [PubMed] [Google Scholar]
- WHEELER A. G., DANSBY D., HAWKINS H. C., PAYNE H. G., WEIKEL J. H., Jr A toxicologic and hematologic evaluation of cyclophosphamide (Cytoxan) in experimental animals. Toxicol Appl Pharmacol. 1962 May;4:324–343. doi: 10.1016/0041-008x(62)90044-3. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- ZAUN H. TIEREXPERIMENTELLE UNTERSUCHUNGEN ZUR PATHOPHYSIOLOGIE DER "GEMISCHTEN ALOPECIE". Arch Klin Exp Dermatol. 1964 Nov 24;221:75–84. [PubMed] [Google Scholar]
- Zaun H. Pathologische Reaktionen am Haarfollikel. Klinische und experimentelle Untersuchungen über Art, Ursache und Bedeutung von Normabweichungen im Haarwurzelmuster. Ann Univ Sarav Med. 1967;14(3):148–223. [PubMed] [Google Scholar]