Abstract
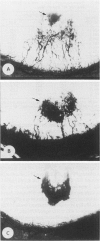
Psoriasis is a common inherited skin disease that is characterized by hyperproliferation of epidermal keratinocytes and excessive dermal angiogenesis. A growing body of evidence supports a key pathogenetic role for activated keratinocytes in the angiogenic response that accompanies psoriasis. We investigated the role of psoriatic epidermis in the aberrant expression of angiogenesis by examining the ability of pure populations of multipassaged keratinocytes obtained from the skin of normal individuals and psoriatic patients to induce angiogenesis in vivo in the rat corneal bioassay and endothelial cell chemotaxis in vitro. Media conditioned by keratinocytes from psoriatic patients, including both symptomless skin and psoriatic plaques, induced vigorous angiogenic responses in over 90% of corneas tested and potently stimulated directional migration of capillary endothelial cells in vitro. In contrast, conditioned medium from normal keratinocyte cultures was weakly positive in less than 10% of corneas assayed and failed to stimulate endothelial cell chemotaxis. Furthermore, keratinocytes from psoriatic skin exhibited a 10- to 20-fold increase in interleukin-8 production and a seven-fold reduction in thrombospondin-1 production. The angiogenic activity present in keratinocyte-conditioned media from psoriatic patients was suppressed by adding either highly purified thrombospondin-1 (125 ng) or following the addition of either normal keratinocyte-conditioned media or neutralizing interleukin-8 antibody. We conclude that psoriatic keratinocytes are phenotypically different from normal keratinocytes with respect to their angiogenic capacity and that this aberrant phenotype is attributable to a defect in the overproduction of interleukin-8 and a deficiency in the production of the angiogenesis inhibitor thrombospondin-1.
Full text
PDF


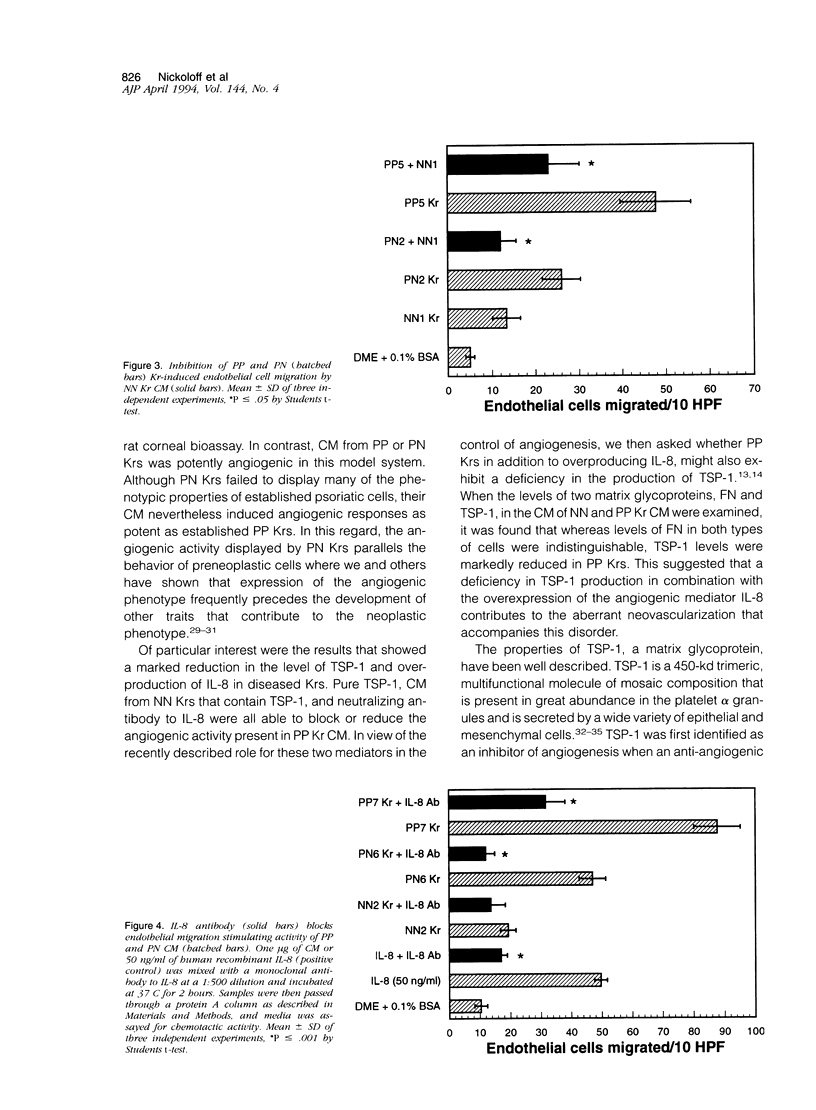


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker B. S., Powles A. V., Valdimarsson H., Fry L. An altered response by psoriatic keratinocytes to gamma interferon. Scand J Immunol. 1988 Dec;28(6):735–740. doi: 10.1111/j.1365-3083.1988.tb01507.x. [DOI] [PubMed] [Google Scholar]
- Braverman I. M., Yen A. Ultrastructure of the capillary loops in the dermal papillae of psoriasis. J Invest Dermatol. 1977 Jan;68(1):53–60. doi: 10.1111/1523-1747.ep12485169. [DOI] [PubMed] [Google Scholar]
- Brem S. S., Jensen H. M., Gullino P. M. Angiogenesis as a marker of preneoplastic lesions of the human breast. Cancer. 1978 Jan;41(1):239–244. doi: 10.1002/1097-0142(197801)41:1<239::aid-cncr2820410133>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- DiPietro L. A., Polverini P. J. Angiogenic macrophages produce the angiogenic inhibitor thrombospondin 1. Am J Pathol. 1993 Sep;143(3):678–684. [PMC free article] [PubMed] [Google Scholar]
- Dixit V. M., Grant G. A., Santoro S. A., Frazier W. A. Isolation and characterization of a heparin-binding domain from the amino terminus of platelet thrombospondin. J Biol Chem. 1984 Aug 25;259(16):10100–10105. [PubMed] [Google Scholar]
- Farber E. M., Nall M. L., Watson W. Natural history of psoriasis in 61 twin pairs. Arch Dermatol. 1974 Feb;109(2):207–211. [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in psoriasis: therapeutic implications. J Invest Dermatol. 1972 Jul;59(1):40–43. doi: 10.1111/1523-1747.ep12625746. [DOI] [PubMed] [Google Scholar]
- Folkman J., Cotran R. Relation of vascular proliferation to tumor growth. Int Rev Exp Pathol. 1976;16:207–248. [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990 Jan 3;82(1):4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Frazier W. A. Thrombospondin: a modular adhesive glycoprotein of platelets and nucleated cells. J Cell Biol. 1987 Aug;105(2):625–632. doi: 10.1083/jcb.105.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier W. A. Thrombospondins. Curr Opin Cell Biol. 1991 Oct;3(5):792–799. doi: 10.1016/0955-0674(91)90052-z. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Fincham N. J., Bird C. R., Wadhwa M., Meager A., Cartwright J. E., Camp R. D. Cytokines in skin lesions of psoriasis. Cytokine. 1990 Jan;2(1):68–75. doi: 10.1016/1043-4666(90)90045-u. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Gullino P. M. Neovascularization induced by intraocular xenografts of normal, preneoplastic, and neoplastic mouse mammary tissues. J Natl Cancer Inst. 1976 Feb;56(2):305–318. doi: 10.1093/jnci/56.2.305. [DOI] [PubMed] [Google Scholar]
- Good D. J., Polverini P. J., Rastinejad F., Le Beau M. M., Lemons R. S., Frazier W. A., Bouck N. P. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruela-Arispe M. L., Bornstein P., Sage H. Thrombospondin exerts an antiangiogenic effect on cord formation by endothelial cells in vitro. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5026–5030. doi: 10.1073/pnas.88.11.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Kunkel S. L., Harlow L. A., DiPietro L. A., Elner V. M., Elner S. G., Strieter R. M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992 Dec 11;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Leibovich S. J. Stimulation of neovascularization by human rheumatoid synovial tissue macrophages. Arthritis Rheum. 1986 Apr;29(4):471–479. doi: 10.1002/art.1780290403. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Lawler J. The structural and functional properties of thrombospondin. Blood. 1986 May;67(5):1197–1209. [PubMed] [Google Scholar]
- Malhotra R., Stenn K. S., Fernandez L. A., Braverman I. M. Angiogenic properties of normal and psoriatic skin associate with epidermis, not dermis. Lab Invest. 1989 Aug;61(2):162–165. [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Oppenheim J. J. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989 Nov;1(1):2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Karabin G. D., Barker J. N., Griffiths C. E., Sarma V., Mitra R. S., Elder J. T., Kunkel S. L., Dixit V. M. Cellular localization of interleukin-8 and its inducer, tumor necrosis factor-alpha in psoriasis. Am J Pathol. 1991 Jan;138(1):129–140. [PMC free article] [PubMed] [Google Scholar]
- Nickoloff B. J., Mitra R. S., Elder J. T., Fisher G. J., Voorhees J. J. Decreased growth inhibition by recombinant gamma interferon is associated with increased transforming growth factor-alpha production in keratinocytes cultured from psoriatic lesions. Br J Dermatol. 1989 Aug;121(2):161–174. doi: 10.1111/j.1365-2133.1989.tb01795.x. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Mitra R. S., Riser B. L., Dixit V. M., Varani J. Modulation of keratinocyte motility. Correlation with production of extracellular matrix molecules in response to growth promoting and antiproliferative factors. Am J Pathol. 1988 Sep;132(3):543–551. [PMC free article] [PubMed] [Google Scholar]
- Nickoloff B. J., Riser B. L., Mitra R. S., Dixit V. M., Varani J. Inhibitory effect of gamma interferon on cultured human keratinocyte thrombospondin production, distribution, and biologic activities. J Invest Dermatol. 1988 Sep;91(3):213–218. doi: 10.1111/1523-1747.ep12465005. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J. The cytokine network in psoriasis. Arch Dermatol. 1991 Jun;127(6):871–884. [PubMed] [Google Scholar]
- O'Shea K. S., Dixit V. M. Unique distribution of the extracellular matrix component thrombospondin in the developing mouse embryo. J Cell Biol. 1988 Dec;107(6 Pt 2):2737–2748. doi: 10.1083/jcb.107.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent D., Bernard B. A., Desbas C., Heenen M., Darmon M. Y. Spreading of psoriatic plaques: alteration of epidermal differentiation precedes capillary leakiness and anomalies in vascular morphology. J Invest Dermatol. 1990 Sep;95(3):333–340. doi: 10.1111/1523-1747.ep12485084. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Leibovich S. J. Induction of neovascularization in vivo and endothelial proliferation in vitro by tumor-associated macrophages. Lab Invest. 1984 Dec;51(6):635–642. [PubMed] [Google Scholar]
- Polverini P. J., Solt D. B. Expression of the angiogenic phenotype by a subpopulation of keratinocytes derived from 7,12-dimethylbenz[a]anthracene-initiated hamster buccal pouch epithelium. Carcinogenesis. 1988 Jan;9(1):117–122. doi: 10.1093/carcin/9.1.117. [DOI] [PubMed] [Google Scholar]
- Rastinejad F., Polverini P. J., Bouck N. P. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell. 1989 Feb 10;56(3):345–355. doi: 10.1016/0092-8674(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Sage E. H., Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991 Aug 15;266(23):14831–14834. [PubMed] [Google Scholar]
- Sage H., Bornstein P. Endothelial cells from umbilical vein and a hemangioendothelioma secrete basement membrane largely to the exclusion of interstitial procollagens. Arteriosclerosis. 1982 Jan-Feb;2(1):27–36. doi: 10.1161/01.atv.2.1.27. [DOI] [PubMed] [Google Scholar]
- Sticherling M., Bornscheuer E., Schröder J. M., Christophers E. Localization of neutrophil-activating peptide-1/interleukin-8-immunoreactivity in normal and psoriatic skin. J Invest Dermatol. 1991 Jan;96(1):26–30. doi: 10.1111/1523-1747.ep12514689. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Elner V. M., Martonyi C. L., Koch A. E., Polverini P. J., Elner S. G. Interleukin-8. A corneal factor that induces neovascularization. Am J Pathol. 1992 Dec;141(6):1279–1284. [PMC free article] [PubMed] [Google Scholar]
- Tolsma S. S., Volpert O. V., Good D. J., Frazier W. A., Polverini P. J., Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993 Jul;122(2):497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J. E., Jr Angiogenesis in normal and psoriatic skin. Lab Invest. 1989 Aug;61(2):139–142. [PubMed] [Google Scholar]