Abstract
The formation of hepatic granulomas around persistently deposited Schistosoma mansoni eggs leads to parenchymal damage, ongoing fibrosis, and ultimate loss of liver function. In this study, the production of macrophage inflammatory protein-1 alpha (MIP-1) and monocyte chemoattractant protein-1 (MCP-1) by granuloma fibroblasts was examined to establish the potential contribution of intragranuloma fibroblasts to the maintenance of the chronic inflammation. Isolated fibroblasts from dispersed acute infection hepatic granulomas were grown in tissue culture for 3 to 4 weeks and used on the third or fourth passage. We initially surveyed fibroblasts for production of MIP-1 and MCP-1 by reverse transcription-polymerase chain reaction (RT-PCR) after stimulation with interleukin (IL)-1, tumor necrosis factor, interferon (IFN)-gamma, IL-4, or IL-10: cytokines found within the granuloma. These studies demonstrated constitutive expression of MCP-1 and differential up-regulation of MIP-1 on cytokine stimulation. Protein expression was then verified by immunohistochemical localization of MIP-1 and MCP-1 in paraformaldehyde-fixed fibroblasts and by direct quantitation of MIP-1 and MCP-1 in culture supernatants by specific ELISAs. These studies demonstrated constitutive expression of MCP-1 in unstimulated and cytokine-stimulated granuloma fibroblasts. In contrast, IL-1 (0.1 to 2.5 ng/ml), IFN-gamma (10 micrograms/ml), and IL-10 (2.5 to 10 ng/ml) were able to induce the significant production of MIP-1 by the granuloma fibroblasts. Interestingly, normal noninflammatory fibroblasts from uninfected mice showed no significant production of MIP-1 or MCP-1 in response to these cytokines. These results suggest that granuloma fibroblasts may be phenotypically altered compared with normal fibroblasts and have a significant role in leukocyte recruitment, granuloma growth, and maintenance of the egg-induced lesion.
Full text
PDF



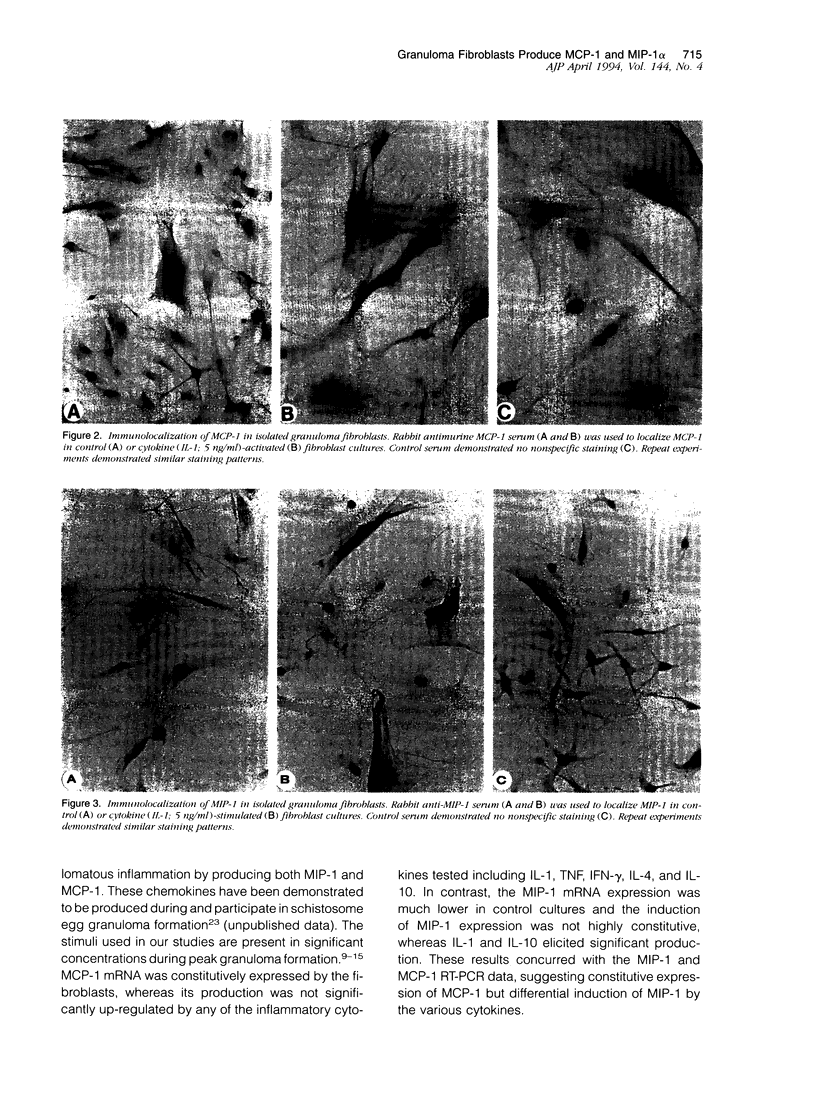
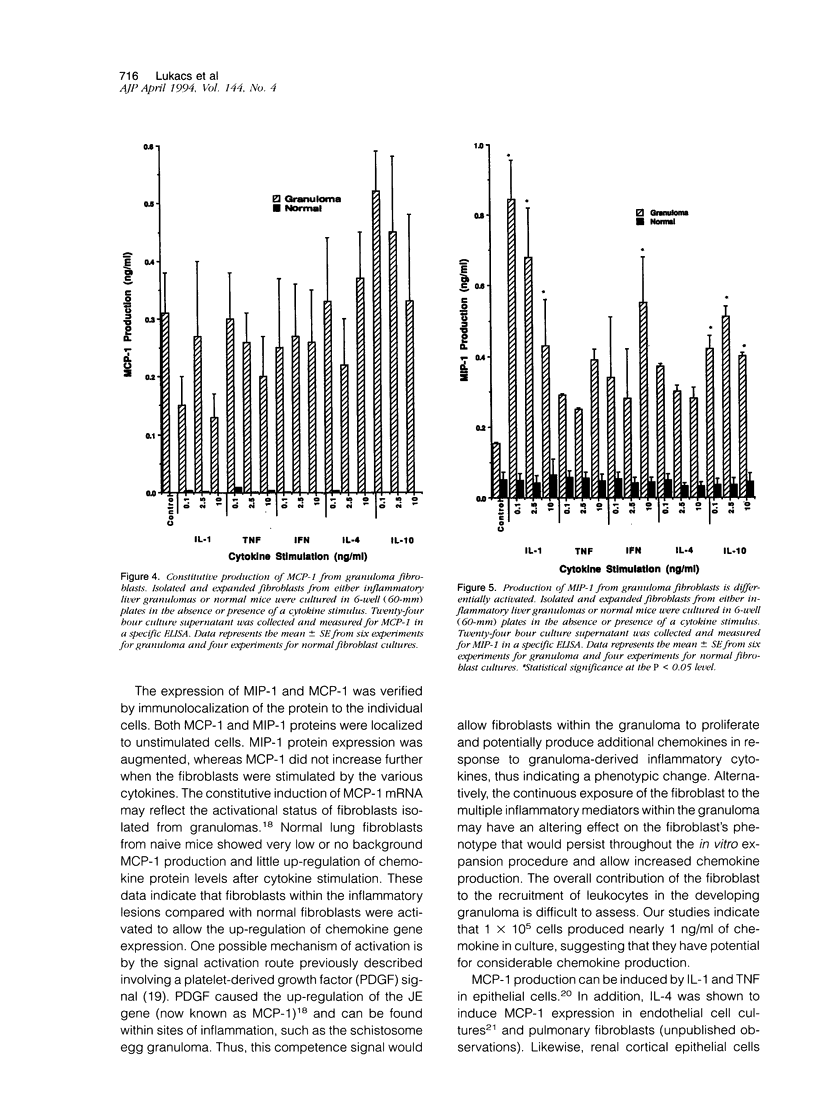


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boros D. L. Immunopathology of Schistosoma mansoni infection. Clin Microbiol Rev. 1989 Jul;2(3):250–269. doi: 10.1128/cmr.2.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros D. L., Pelley R. P., Warren K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J Immunol. 1975 May;114(5):1437–1441. [PubMed] [Google Scholar]
- Callahan M., Cochran B. H., Stiles C. D. The PDGF-inducible 'competence genes': intracellular mediators of the mitogenic response. Ciba Found Symp. 1985;116:87–97. doi: 10.1002/9780470720974.ch6. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L., David C. S. Regulation of granulomatous inflammation in murine schistosomiasis. In vitro characterization of T lymphocyte subsets involved in the production and suppression of migration inhibition factor. J Exp Med. 1980 Jun 1;151(6):1398–1412. doi: 10.1084/jem.151.6.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L. Modulation of granulomatous hypersensitivity. I. Characterization of T lymphocytes involved in the adoptive suppression of granuloma formation in Schistosoma mansoni-infected mice. J Immunol. 1979 Sep;123(3):1409–1414. [PubMed] [Google Scholar]
- Chensue S. W., Otterness I. G., Higashi G. I., Forsch C. S., Kunkel S. L. Monokine production by hypersensitivity (Schistosoma mansoni egg) and foreign body (Sephadex bead)-type granuloma macrophages. Evidence for sequential production of IL-1 and tumor necrosis factor. J Immunol. 1989 Feb 15;142(4):1281–1286. [PubMed] [Google Scholar]
- Chensue S. W., Terebuh P. D., Warmington K. S., Hershey S. D., Evanoff H. L., Kunkel S. L., Higashi G. I. Role of IL-4 and IFN-gamma in Schistosoma mansoni egg-induced hypersensitivity granuloma formation. Orchestration, relative contribution, and relationship to macrophage function. J Immunol. 1992 Feb 1;148(3):900–906. [PubMed] [Google Scholar]
- Davatelis G., Wolpe S. D., Sherry B., Dayer J. M., Chicheportiche R., Cerami A. Macrophage inflammatory protein-1: a prostaglandin-independent endogenous pyrogen. Science. 1989 Feb 24;243(4894 Pt 1):1066–1068. doi: 10.1126/science.2646711. [DOI] [PubMed] [Google Scholar]
- Evanoff H. L., Burdick M. D., Moore S. A., Kunkel S. L., Strieter R. M. A sensitive ELISA for the detection of human monocyte chemoattractant protein-1 (MCP-1). Immunol Invest. 1992 Feb;21(1):39–45. doi: 10.3109/08820139209069361. [DOI] [PubMed] [Google Scholar]
- Fahey T. J., 3rd, Tracey K. J., Tekamp-Olson P., Cousens L. S., Jones W. G., Shires G. T., Cerami A., Sherry B. Macrophage inflammatory protein 1 modulates macrophage function. J Immunol. 1992 May 1;148(9):2764–2769. [PubMed] [Google Scholar]
- Grzych J. M., Pearce E., Cheever A., Caulada Z. A., Caspar P., Heiny S., Lewis F., Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991 Feb 15;146(4):1322–1327. [PubMed] [Google Scholar]
- Jiang Y., Beller D. I., Frendl G., Graves D. T. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992 Apr 15;148(8):2423–2428. [PubMed] [Google Scholar]
- Lukacs N. W., Boros D. L. Lymphokine regulation of granuloma formation in murine schistosomiasis mansoni. Clin Immunol Immunopathol. 1993 Jul;68(1):57–63. doi: 10.1006/clin.1993.1095. [DOI] [PubMed] [Google Scholar]
- Lukacs N. W., Boros D. L. Utilization of fractionated soluble egg antigens reveals selectively modulated granulomatous and lymphokine responses during murine schistosomiasis mansoni. Infect Immun. 1992 Aug;60(8):3209–3216. doi: 10.1128/iai.60.8.3209-3216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs N. W., Kunkel S. L., Strieter R. M., Warmington K., Chensue S. W. The role of macrophage inflammatory protein 1 alpha in Schistosoma mansoni egg-induced granulomatous inflammation. J Exp Med. 1993 Jun 1;177(6):1551–1559. doi: 10.1084/jem.177.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Moore K. W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991 Mar;12(3):A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Prakash S., Postlethwaite A. E., Wyler D. J. Alterations in influence of granuloma-derived cytokines on fibrogenesis in the course of murine Schistosoma mansoni infection. Hepatology. 1991 May;13(5):970–976. [PubMed] [Google Scholar]
- Prakash S., Postlethwaite A. E., Wyler D. J. Alterations in influence of granuloma-derived cytokines on fibrogenesis in the course of murine Schistosoma mansoni infection. Hepatology. 1991 May;13(5):970–976. [PubMed] [Google Scholar]
- Ragheb S., Boros D. L. Characterization of granuloma T lymphocyte function from Schistosoma mansoni-infected mice. J Immunol. 1989 May 1;142(9):3239–3246. [PubMed] [Google Scholar]
- Rollins B. J., Morrison E. D., Stiles C. D. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3738–3742. doi: 10.1073/pnas.85.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Pober J. S. Interleukin-4 induces the synthesis and secretion of MCP-1/JE by human endothelial cells. Am J Pathol. 1991 Jun;138(6):1315–1319. [PMC free article] [PubMed] [Google Scholar]
- Schall T. J. Biology of the RANTES/SIS cytokine family. Cytokine. 1991 May;3(3):165–183. doi: 10.1016/1043-4666(91)90013-4. [DOI] [PubMed] [Google Scholar]
- Sher A., Fiorentino D., Caspar P., Pearce E., Mosmann T. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol. 1991 Oct 15;147(8):2713–2716. [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Phan S. H., Rollins B. J., Strieter R. M. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J Biol Chem. 1991 May 25;266(15):9912–9918. [PubMed] [Google Scholar]
- Standiford T. J., Rolfe M. R., Kunkel S. L., Lynch J. P., 3rd, Becker F. S., Orringer M. B., Phan S., Strieter R. M. Altered production and regulation of monocyte chemoattractant protein-1 from pulmonary fibroblasts isolated from patients with idiopathic pulmonary fibrosis. Chest. 1993 Feb;103(2 Suppl):121S–121S. doi: 10.1378/chest.103.2_supplement.121s. [DOI] [PubMed] [Google Scholar]
- Wolpe S. D., Cerami A. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J. 1989 Dec;3(14):2565–2573. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- Wyler D. J., Postlethwaite A. E. Fibroblast stimulation in schistosomiasis. IV. Isolated egg granulomas elaborate a fibroblast chemoattractant in vitro. J Immunol. 1983 Mar;130(3):1371–1375. [PubMed] [Google Scholar]
- Wyler D. J., Wahl S. M., Cheever A. W., Wahl L. M. Fibroblast stimulation in schistosomiasis. I. Stimulation in vitro of fibroblasts by soluble products of egg granulomas. J Infect Dis. 1981 Sep;144(3):254–262. doi: 10.1093/infdis/144.3.254. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Boros D. L. IL-4 influences IL-2 production and granulomatous inflammation in murine schistosomiasis mansoni. J Immunol. 1992 Dec 1;149(11):3659–3664. [PubMed] [Google Scholar]





