Abstract
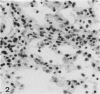
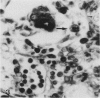
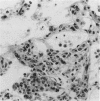
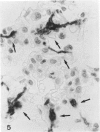
The majority of fibroblasts in alveolar septa are characterized by the presence of cytoplasmic bundles of microfilaments that contain cytoplasmic actin isoforms; these cells have been named contractile interstitial cells or V-type myofibroblasts. In the rat, they express desmin as intermediate filament protein. In this study, we explored the possibility that modulation and replication of such septal fibroblasts result in the appearance of alpha-smooth muscle (alpha-SM) actin-positive myofibroblasts, typical of lung fibrosis. Experimental pulmonary fibrosis was produced by a unique intratracheal instillation of bleomycin to 28 rats. Eight additional rats used as controls received the equivalent volume of saline. Paraffin and frozen sections of lungs were examined at days 1, 3, 5 and 7 after treatment. Microfilaments and intermediate filaments were stained using antibodies against total actin, alpha-SM actin, desmin, vimentin, keratin, and SM myosin. Electron microscopic labeling of desmin and alpha-SM actin using immunogold technique was done on Lowicryl K4M resin-embedded specimens. alpha-SM actin appeared in desmin-positive alveolar fibroblasts as early as 24 hours after intratracheal bleomycin instillation; the modulation of alpha-SM actin in these cells was preceded by a lymphomonocytic infiltration of alveolar septa. Twenty-four hours to 3 days after bleomycin administration, a proliferation of alveolar myofibroblasts occurred. Fibrosis with laying down of collagen fibers took place after the above mentioned cellular modifications. Our results support the view that septal fibroblastic cells can modulate into typical alpha-SM actin-containing myofibroblasts during experimental bleomycin-induced pulmonary fibrosis. In such a modulation a possible role of cytokines, particularly of transforming growth factor-beta, is considered.
Full text
PDF



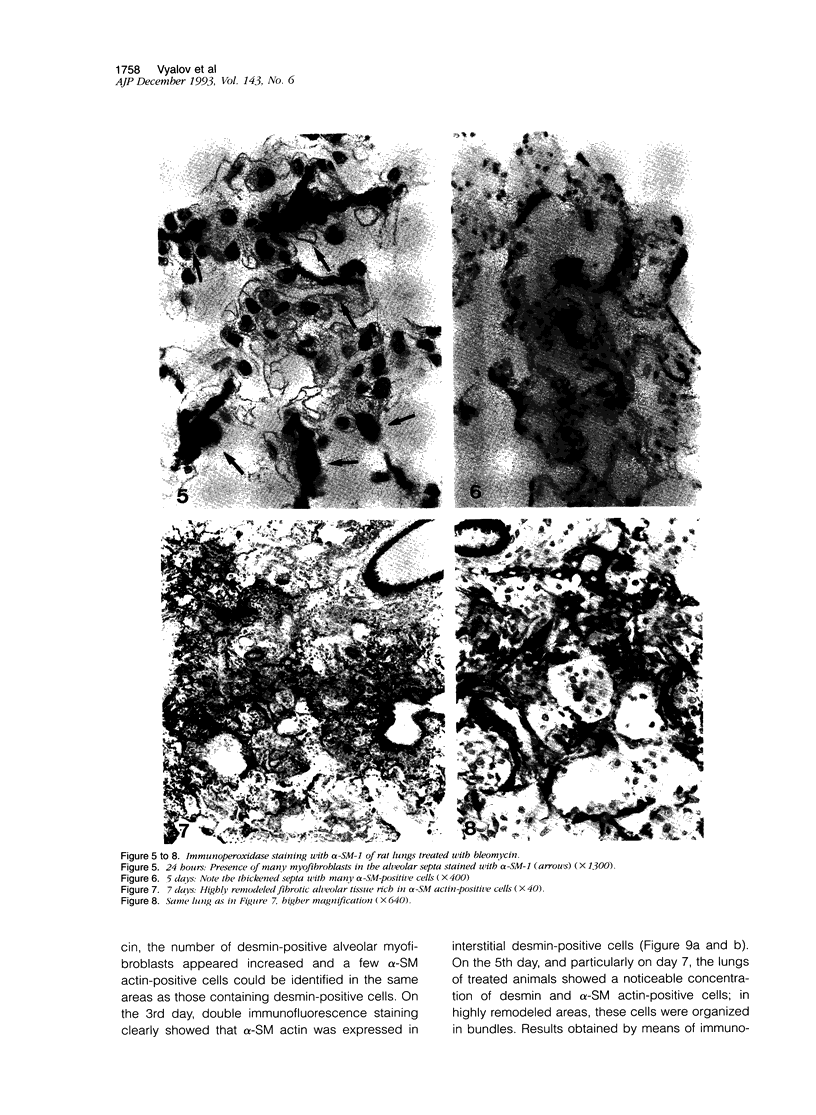
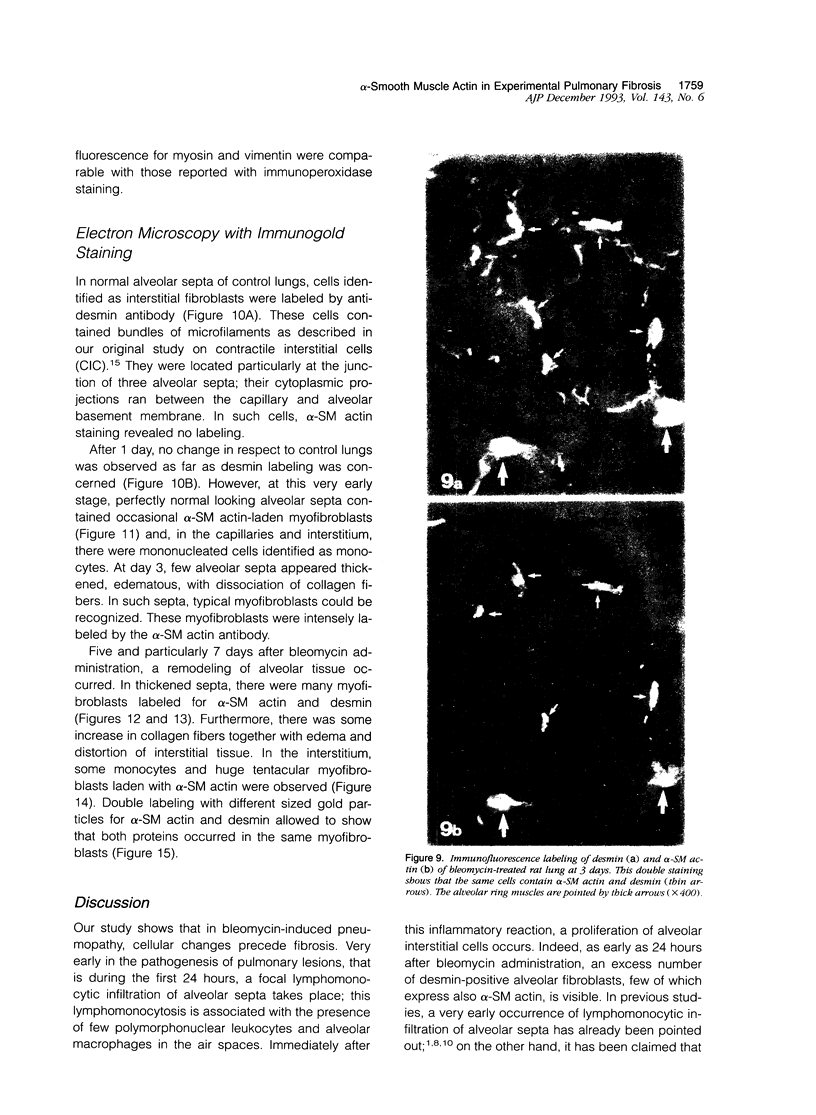
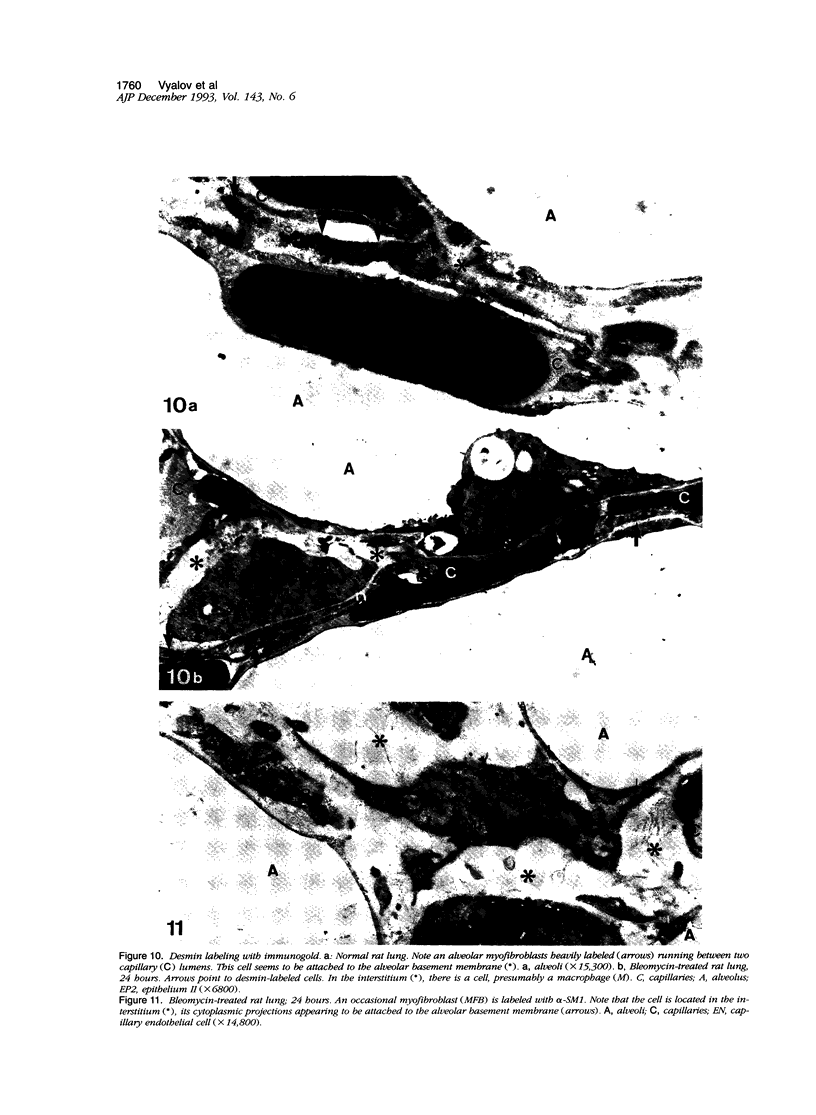
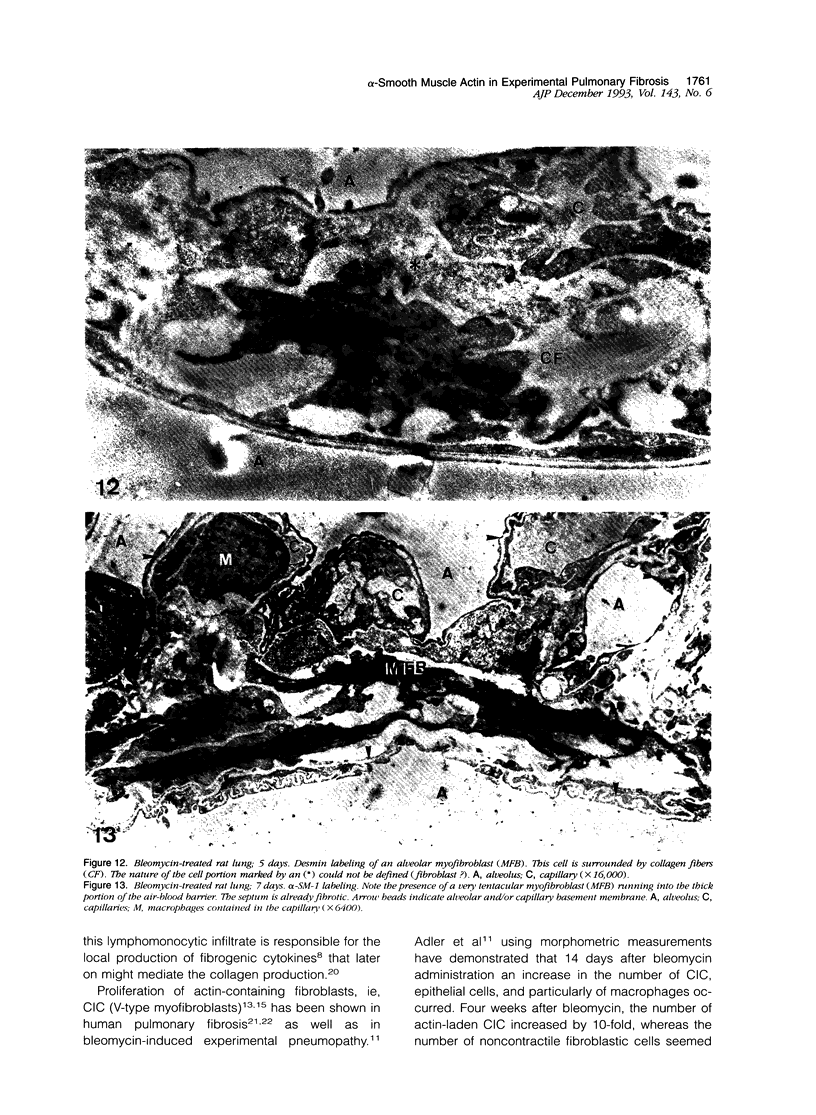
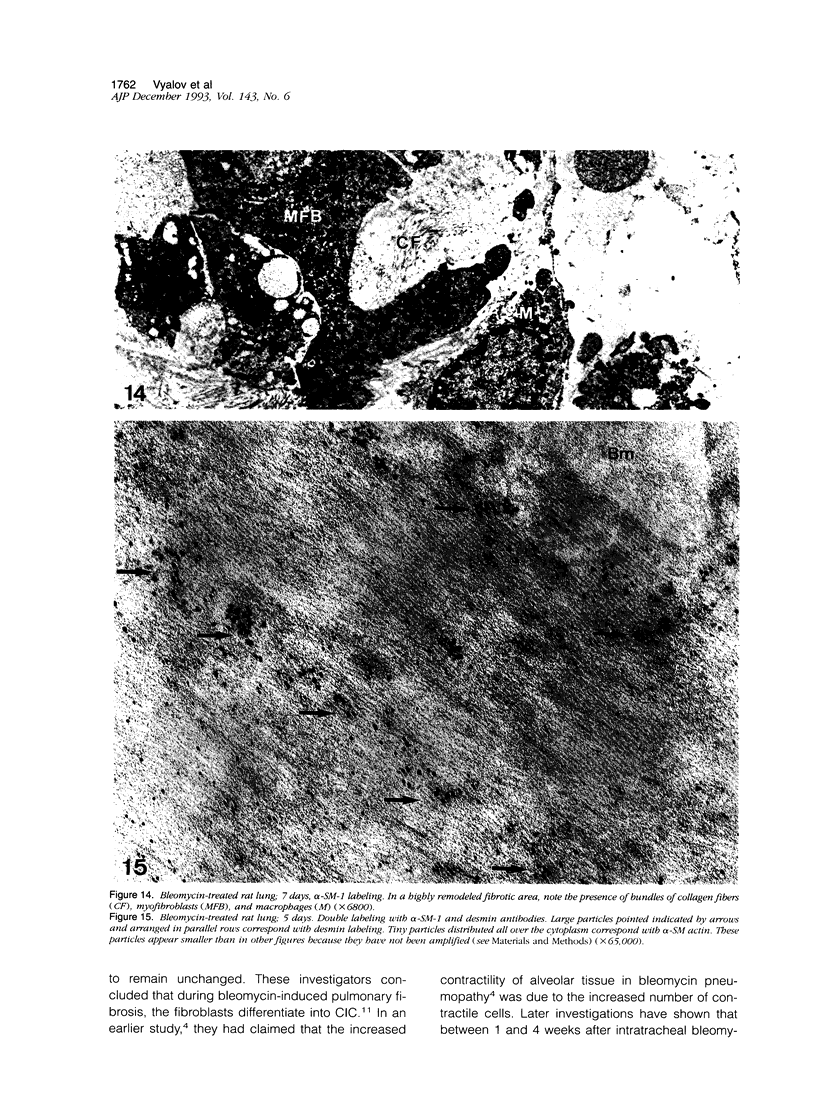



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler K. B., Callahan L. M., Evans J. N. Cellular alterations in the alveolar wall in bleomycin-induced pulmonary fibrosis in rats. An ultrastructural morphometric study. Am Rev Respir Dis. 1986 Jun;133(6):1043–1048. doi: 10.1164/arrd.1986.133.6.1043. [DOI] [PubMed] [Google Scholar]
- Adler K. B., Low R. B., Leslie K. O., Mitchell J., Evans J. N. Contractile cells in normal and fibrotic lung. Lab Invest. 1989 Apr;60(4):473–485. [PubMed] [Google Scholar]
- Bachem M. G., Sell K. M., Melchior R., Kropf J., Eller T., Gressner A. M. Tumor necrosis factor alpha (TNF alpha) and transforming growth factor beta 1 (TGF beta 1) stimulate fibronectin synthesis and the transdifferentiation of fat-storing cells in the rat liver into myofibroblasts. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63(2):123–130. doi: 10.1007/BF02899251. [DOI] [PubMed] [Google Scholar]
- Benzonana G., Skalli O., Gabbiani G. Correlation between the distribution of smooth muscle or non muscle myosins and alpha-smooth muscle actin in normal and pathological soft tissues. Cell Motil Cytoskeleton. 1988;11(4):260–274. doi: 10.1002/cm.970110405. [DOI] [PubMed] [Google Scholar]
- Björkerud S. Effects of transforming growth factor-beta 1 on human arterial smooth muscle cells in vitro. Arterioscler Thromb. 1991 Jul-Aug;11(4):892–902. [PubMed] [Google Scholar]
- Border W. A., Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992 Jul;90(1):1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. B. Possible mechanisms of bleomycin-induced fibrosis. Clin Chest Med. 1990 Mar;11(1):21–30. [PubMed] [Google Scholar]
- Danscher G. Localization of gold in biological tissue. A photochemical method for light and electronmicroscopy. Histochemistry. 1981;71(1):81–88. doi: 10.1007/BF00592572. [DOI] [PubMed] [Google Scholar]
- Darby I., Skalli O., Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990 Jul;63(1):21–29. [PubMed] [Google Scholar]
- Desmoulière A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993 Jul;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane K., McReynolds R. A., Wilson F. J. Immunofluorescence localization of contractile proteins in the rat lung following bleomycin injury. Histochem J. 1983 Jan;15(1):82–88. doi: 10.1007/BF01006074. [DOI] [PubMed] [Google Scholar]
- Evans J. N., Kelley J., Low R. B., Adler K. B. Increased contractility of isolated lung parenchyma in an animal model of pulmonary fibrosis induced by bleomycin. Am Rev Respir Dis. 1982 Jan;125(1):89–94. doi: 10.1164/arrd.1982.125.1.89. [DOI] [PubMed] [Google Scholar]
- Hay J., Shahzeidi S., Laurent G. Mechanisms of bleomycin-induced lung damage. Arch Toxicol. 1991;65(2):81–94. doi: 10.1007/BF02034932. [DOI] [PubMed] [Google Scholar]
- Kapanci Y., Assimacopoulos A., Irle C., Zwahlen A., Gabbiani G. "Contractile interstitial cells" in pulmonary alveolar septa: a possible regulator of ventilation-perfusion ratio? Ultrastructural, immunofluorescence, and in vitro studies. J Cell Biol. 1974 Feb;60(2):375–392. doi: 10.1083/jcb.60.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapanci Y., Burgan S., Pietra G. G., Conne B., Gabbiani G. Modulation of actin isoform expression in alveolar myofibroblasts (contractile interstitial cells) during pulmonary hypertension. Am J Pathol. 1990 Apr;136(4):881–889. [PMC free article] [PubMed] [Google Scholar]
- Kapanci Y., Ribaux C., Chaponnier C., Gabbiani G. Cytoskeletal features of alveolar myofibroblasts and pericytes in normal human and rat lung. J Histochem Cytochem. 1992 Dec;40(12):1955–1963. doi: 10.1177/40.12.1333502. [DOI] [PubMed] [Google Scholar]
- Kelley J., Kovacs E. J., Nicholson K., Fabisiak J. P. Transforming growth factor-beta production by lung macrophages and fibroblasts. Chest. 1991 Mar;99(3 Suppl):85S–86S. [PubMed] [Google Scholar]
- Khalil N., Bereznay O., Sporn M., Greenberg A. H. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989 Sep 1;170(3):727–737. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs E. J. Fibrogenic cytokines: the role of immune mediators in the development of scar tissue. Immunol Today. 1991 Jan;12(1):17–23. doi: 10.1016/0167-5699(91)90107-5. [DOI] [PubMed] [Google Scholar]
- Lazenby A. J., Crouch E. C., McDonald J. A., Kuhn C., 3rd Remodeling of the lung in bleomycin-induced pulmonary fibrosis in the rat. An immunohistochemical study of laminin, type IV collagen, and fibronectin. Am Rev Respir Dis. 1990 Jul;142(1):206–214. doi: 10.1164/ajrccm/142.1.206. [DOI] [PubMed] [Google Scholar]
- Lazo J. S., Hoyt D. G., Sebti S. M., Pitt B. R. Bleomycin: a pharmacologic tool in the study of the pathogenesis of interstitial pulmonary fibrosis. Pharmacol Ther. 1990;47(3):347–358. doi: 10.1016/0163-7258(90)90061-6. [DOI] [PubMed] [Google Scholar]
- Leslie K. O., Mitchell J., Low R. Lung myofibroblasts. Cell Motil Cytoskeleton. 1992;22(2):92–98. doi: 10.1002/cm.970220203. [DOI] [PubMed] [Google Scholar]
- Low R. B., Woodcock-Mitchell J., Evans J. N., Adler K. B. Actin content of normal and of bleomycin-fibrotic rat lung. Am Rev Respir Dis. 1984 Feb;129(2):311–316. [PubMed] [Google Scholar]
- Mitchell J., Woodcock-Mitchell J., Reynolds S., Low R., Leslie K., Adler K., Gabbiani G., Skalli O. Alpha-smooth muscle actin in parenchymal cells of bleomycin-injured rat lung. Lab Invest. 1989 May;60(5):643–650. [PubMed] [Google Scholar]
- Piguet P. F., Collart M. A., Grau G. E., Kapanci Y., Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med. 1989 Sep 1;170(3):655–663. doi: 10.1084/jem.170.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubbia-Brandt L., Sappino A. P., Gabbiani G. Locally applied GM-CSF induces the accumulation of alpha-smooth muscle actin containing myofibroblasts. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60(2):73–82. doi: 10.1007/BF02899530. [DOI] [PubMed] [Google Scholar]
- Sappino A. P., Schürch W., Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990 Aug;63(2):144–161. [PubMed] [Google Scholar]
- Schmitt-Gräff A., Krüger S., Bochard F., Gabbiani G., Denk H. Modulation of alpha smooth muscle actin and desmin expression in perisinusoidal cells of normal and diseased human livers. Am J Pathol. 1991 May;138(5):1233–1242. [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Pelte M. F., Peclet M. C., Gabbiani G., Gugliotta P., Bussolati G., Ravazzola M., Orci L. Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J Histochem Cytochem. 1989 Mar;37(3):315–321. doi: 10.1177/37.3.2918221. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrall R. S., McCormick J. R., Jack R. M., McReynolds R. A., Ward P. A. Bleomycin-induced pulmonary fibrosis in the rat: inhibition by indomethacin. Am J Pathol. 1979 Apr;95(1):117–130. [PMC free article] [PubMed] [Google Scholar]
- Vyalov S., Desmoulière A., Gabbiani G. GM-CSF-induced granulation tissue formation: relationships between macrophage and myofibroblast accumulation. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63(4):231–239. doi: 10.1007/BF02899267. [DOI] [PubMed] [Google Scholar]
- Woodcock-Mitchell J., Adler K. B., Low R. B. Immunohistochemical identification of cell types in normal and in bleomycin-induced fibrotic rat lung. Cellular origins of interstitial cells. Am Rev Respir Dis. 1984 Nov;130(5):910–916. doi: 10.1164/arrd.1984.130.5.910. [DOI] [PubMed] [Google Scholar]