Abstract
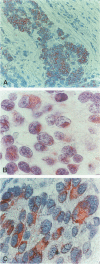
Survival rate in neuroblastoma, a tumor of post-ganglionic sympathetic neuroblasts, correlates with disease stage, tumor histology, and N-myc gene amplification. N-myc amplification is associated with rapid tumor progression and poor survival, but is not present in all cases of poor prognosis neuroblastoma. Moreover, overexpression of N-myc is not sufficient to cause cellular transformation. These data suggest that other genetic factors are important for neuroblastoma development. We investigated the expression of the, bcl-2 proto-oncogene in untreated cases of neuroblastoma. bcl-2 is a novel proto-oncogene that promotes cell growth by inhibiting programmed cell death (apoptosis), a form of cellular demise common during normal neurogenesis. Immunocytochemical localization using a monoclonal anti-bcl-2 antibody revealed that 16 of 40 patient specimens stained positive for bcl-2. bcl-2 was strongly associated with unfavorable histology (P = 0.002) and N-myc gene amplification (P = 0.002) and marginally associated with poor stage disease (P = 0.06). A logistic regression model evaluating the simultaneous association of stage, histology, and N-myc revealed that bcl-2 was most associated with unfavorable histology and N-myc gene amplification. These results support the notion that bcl-2 may play an important role in the genesis or progression of malignant neuroblastoma.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batistatou A., Greene L. A. Aurintricarboxylic acid rescues PC12 cells and sympathetic neurons from cell death caused by nerve growth factor deprivation: correlation with suppression of endonuclease activity. J Cell Biol. 1991 Oct;115(2):461–471. doi: 10.1083/jcb.115.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Bissonnette R. P., Echeverri F., Mahboubi A., Green D. R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992 Oct 8;359(6395):552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- Boyd J. A., Barrett J. C. Genetic and cellular basis of multistep carcinogenesis. Pharmacol Ther. 1990;46(3):469–486. doi: 10.1016/0163-7258(90)90028-z. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Fong C. T. Molecular biology and genetics of human neuroblastoma. Cancer Genet Cytogenet. 1989 Sep;41(2):153–174. doi: 10.1016/0165-4608(89)90243-4. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984 Jun 8;224(4653):1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Brondyk W. H., Boeckman F. A., Fahl W. E. N-myc oncogene enhances mitogenic responsiveness of diploid human fibroblasts to growth factors but fails to immortalize. Oncogene. 1991 Jul;6(7):1269–1276. [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Evans A. E., D'Angio G. J., Randolph J. A proposed staging for children with neuroblastoma. Children's cancer study group A. Cancer. 1971 Feb;27(2):374–378. doi: 10.1002/1097-0142(197102)27:2<374::aid-cncr2820270221>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Garcia I., Martinou I., Tsujimoto Y., Martinou J. C. Prevention of programmed cell death of sympathetic neurons by the bcl-2 proto-oncogene. Science. 1992 Oct 9;258(5080):302–304. doi: 10.1126/science.1411528. [DOI] [PubMed] [Google Scholar]
- Hankin M. H., Schneider B. F., Silver J. Death of the subcallosal glial sling is correlated with formation of the cavum septi pellucidi. J Comp Neurol. 1988 Jun 8;272(2):191–202. doi: 10.1002/cne.902720204. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- KAELLEN B. DEGENERATION AND REGENERATION IN THE VERTEBRATE CENTRAL NERVOUS SYSTEM DURING EMBRYOGENESIS. Prog Brain Res. 1965;14:77–96. [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases: role of the basement membrane. Warner-Lambert Parke-Davis Award lecture. Am J Pathol. 1984 Dec;117(3):339–348. [PMC free article] [PubMed] [Google Scholar]
- Martin D. P., Schmidt R. E., DiStefano P. S., Lowry O. H., Carter J. G., Johnson E. M., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988 Mar;106(3):829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- Nunez G., Seto M., Seremetis S., Ferrero D., Grignani F., Korsmeyer S. J., Dalla-Favera R. Growth- and tumor-promoting effects of deregulated BCL2 in human B-lymphoblastoid cells. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4589–4593. doi: 10.1073/pnas.86.12.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez G., London L., Hockenbery D., Alexander M., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [PubMed] [Google Scholar]
- Oppenheim R. W. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Tse A. G., Cordell J. L., Pulford K. A., Gatter K. C., Mason D. Y. Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990 Aug;137(2):225–232. [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Meister L., Tanaka S., Cuddy M., Yum S., Geyer C., Pleasure D. Differential expression of bcl2 protooncogene in neuroblastoma and other human tumor cell lines of neural origin. Cancer Res. 1991 Dec 15;51(24):6529–6538. [PubMed] [Google Scholar]
- Schwab M., Bishop J. M. Sustained expression of the human protooncogene MYCN rescues rat embryo cells from senescence. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9585–9589. doi: 10.1073/pnas.85.24.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger R. C., Brodeur G. M., Sather H., Dalton A., Siegel S. E., Wong K. Y., Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985 Oct 31;313(18):1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Wada R., Brodeur G. M., Moss T. J., Bjork R. L., Sousa L., Slamon D. J. Expression of N-myc by neuroblastomas with one or multiple copies of the oncogene. Prog Clin Biol Res. 1988;271:41–49. [PubMed] [Google Scholar]
- Shimada H., Chatten J., Newton W. A., Jr, Sachs N., Hamoudi A. B., Chiba T., Marsden H. B., Misugi K. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst. 1984 Aug;73(2):405–416. doi: 10.1093/jnci/73.2.405. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Boone T. C., Seeger R. C., Keith D. E., Chazin V., Lee H. C., Souza L. M. Identification and characterization of the protein encoded by the human N-myc oncogene. Science. 1986 May 9;232(4751):768–772. doi: 10.1126/science.3008339. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Bath M. L., Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990 Nov 22;348(6299):331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Williams G. T. Programmed cell death: apoptosis and oncogenesis. Cell. 1991 Jun 28;65(7):1097–1098. doi: 10.1016/0092-8674(91)90002-g. [DOI] [PubMed] [Google Scholar]
- Wilson L. M., Draper G. J. Neuroblastoma, its natural history and prognosis: a study of 487 cases. Br Med J. 1974 Aug 3;3(5926):301–307. doi: 10.1136/bmj.3.5926.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zutter M., Hockenbery D., Silverman G. A., Korsmeyer S. J. Immunolocalization of the Bcl-2 protein within hematopoietic neoplasms. Blood. 1991 Aug 15;78(4):1062–1068. [PubMed] [Google Scholar]