Abstract
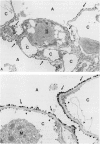
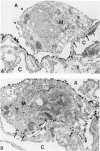
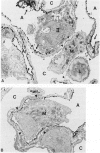
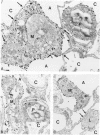
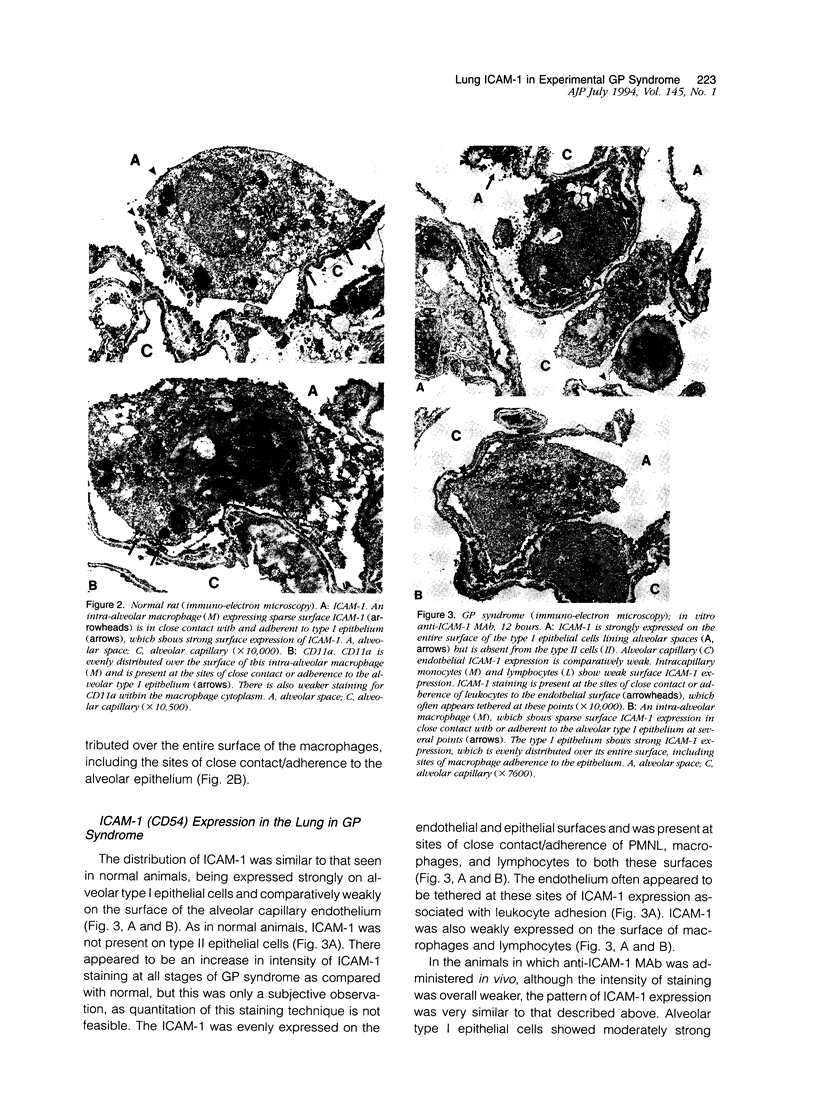
The functional importance of ICAM-1 and its ligands, the beta 2-integrins, in leukocytic accumulation in pulmonary injury has been recently demonstrated in experimental models of lung disease. However, the exact location of these adhesion molecules remains unknown. In the current study we have used immunogold ultrastructural techniques to define the precise location of ICAM-1 in the lung and its interaction with beta 2-integrin expressing leukocytes in the early stages of experimental Goodpasture's (GP) syndrome in the rat. In normal animals there is strong constitutive ICAM-1 expression on the luminal surface of the alveolar epithelium that is confined to type I cells and completely absent from type II cells. Constitutive expression of ICAM-1 on the pulmonary capillary endothelium is comparatively weak. In GP syndrome there is an increase in ICAM-1 expression, which is still confined to the alveolar type I epithelial cells and capillary endothelium. This is associated with an early (1.5 hours) influx of CD18 expressing polymorphonuclear leukocytes, which are seen migrating into alveoli and the pulmonary interstitium. There is a later (6-12 hours) influx of CD11a/CD18 expressing macrophages which are present in the interstitium and in large numbers in the alveolar spaces, where they are very closely apposed to and adherent to the alveolar epithelium. This is the first study to demonstrate the precise ultrastructural location of ICAM-1 in the normal rat lung and in disease. In vivo administered antibody to ICAM-1 gains access to the extravascular sites within the lung, in particular the surface of alveolar type I epithelial cells, and this raises the possibility that beneficial effects of such antibodies may extend beyond their ability to inhibit interactions between leukocytes and endothelial cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton R. W., Rothlein R., Ksiazek J., Kennedy C. The effect of anti-intercellular adhesion molecule-1 on phorbol-ester-induced rabbit lung inflammation. J Immunol. 1989 Aug 15;143(4):1278–1282. [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Christensen P. J., Kim S., Simon R. H., Toews G. B., Paine R., 3rd Differentiation-related expression of ICAM-1 by rat alveolar epithelial cells. Am J Respir Cell Mol Biol. 1993 Jan;8(1):9–15. doi: 10.1165/ajrcmb/8.1.9. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Furie M. B., Tancinco M. C., Smith C. W. Monoclonal antibodies to leukocyte integrins CD11a/CD18 and CD11b/CD18 or intercellular adhesion molecule-1 inhibit chemoattractant-stimulated neutrophil transendothelial migration in vitro. Blood. 1991 Oct 15;78(8):2089–2097. [PubMed] [Google Scholar]
- Horgan M. J., Ge M., Gu J., Rothlein R., Malik A. B. Role of ICAM-1 in neutrophil-mediated lung vascular injury after occlusion and reperfusion. Am J Physiol. 1991 Nov;261(5 Pt 2):H1578–H1584. doi: 10.1152/ajpheart.1991.261.5.H1578. [DOI] [PubMed] [Google Scholar]
- Kavanaugh A. F., Lightfoot E., Lipsky P. E., Oppenheimer-Marks N. Role of CD11/CD18 in adhesion and transendothelial migration of T cells. Analysis utilizing CD18-deficient T cell clones. J Immunol. 1991 Jun 15;146(12):4149–4156. [PubMed] [Google Scholar]
- Lan H. Y., Paterson D. J., Atkins R. C. Initiation and evolution of interstitial leukocytic infiltration in experimental glomerulonephritis. Kidney Int. 1991 Sep;40(3):425–433. doi: 10.1038/ki.1991.229. [DOI] [PubMed] [Google Scholar]
- Lan H. Y., Paterson D. J., Hutchinson P., Atkins R. C. Leukocyte involvement in the pathogenesis of pulmonary injury in experimental Goodpasture's syndrome. Lab Invest. 1991 Mar;64(3):330–338. [PubMed] [Google Scholar]
- Lo S. K., Everitt J., Gu J., Malik A. B. Tumor necrosis factor mediates experimental pulmonary edema by ICAM-1 and CD18-dependent mechanisms. J Clin Invest. 1992 Mar;89(3):981–988. doi: 10.1172/JCI115681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Vaporciyan A. A., Miyasaka M., Tamatani T., Ward P. A. Tumor necrosis factor alpha regulates in vivo intrapulmonary expression of ICAM-1. Am J Pathol. 1993 Jun;142(6):1739–1749. [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Varani J., Warren J. S., Till G. O., Smith C. W., Anderson D. C., Todd R. F., 3rd, Ward P. A. Roles of beta 2 integrins of rat neutrophils in complement- and oxygen radical-mediated acute inflammatory injury. J Immunol. 1992 Mar 15;148(6):1847–1857. [PubMed] [Google Scholar]
- Mulligan M. S., Wilson G. P., Todd R. F., Smith C. W., Anderson D. C., Varani J., Issekutz T. B., Miyasaka M., Tamatani T., Myasaka M. Role of beta 1, beta 2 integrins and ICAM-1 in lung injury after deposition of IgG and IgA immune complexes. J Immunol. 1993 Mar 15;150(6):2407–2417. [PubMed] [Google Scholar]
- Pardi R., Inverardi L., Bender J. R. Regulatory mechanisms in leukocyte adhesion: flexible receptors for sophisticated travelers. Immunol Today. 1992 Jun;13(6):224–230. doi: 10.1016/0167-5699(92)90159-5. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Berry D. T., Schmitt F. A., Magan L. K., Gerhardstein D. C., Cook Y. R. Sleep-disordered breathing in the healthy elderly. Clinically significant? Chest. 1992 Feb;101(2):345–349. doi: 10.1378/chest.101.2.345. [DOI] [PubMed] [Google Scholar]
- Queluz T. H., Pawlowski I., Brunda M. J., Brentjens J. R., Vladutiu A. O., Andres G. Pathogenesis of an experimental model of Goodpasture's hemorrhagic pneumonitis. J Clin Invest. 1990 May;85(5):1507–1515. doi: 10.1172/JCI114597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltini C., Hemler M. E., Crystal R. G. T lymphocytes compartmentalized on the epithelial surface of the lower respiratory tract express the very late activation antigen complex VLA-1. Clin Immunol Immunopathol. 1988 Feb;46(2):221–233. doi: 10.1016/0090-1229(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Seekamp A., Mulligan M. S., Till G. O., Smith C. W., Miyasaka M., Tamatani T., Todd R. F., 3rd, Ward P. A. Role of beta 2 integrins and ICAM-1 in lung injury following ischemia-reperfusion of rat hind limbs. Am J Pathol. 1993 Aug;143(2):464–472. [PMC free article] [PubMed] [Google Scholar]
- Shappell S. B., Toman C., Anderson D. C., Taylor A. A., Entman M. L., Smith C. W. Mac-1 (CD11b/CD18) mediates adherence-dependent hydrogen peroxide production by human and canine neutrophils. J Immunol. 1990 Apr 1;144(7):2702–2711. [PubMed] [Google Scholar]
- Shijubo N., Imai K., Aoki S., Hirasawa M., Sugawara H., Koba H., Tsujisaki M., Sugiyama T., Hinoda Y., Yachi A. Circulating intercellular adhesion molecule-1 (ICAM-1) antigen in sera of patients with idiopathic pulmonary fibrosis. Clin Exp Immunol. 1992 Jul;89(1):58–62. doi: 10.1111/j.1365-2249.1992.tb06877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. H., DeHart P. D., Todd R. F., 3rd Neutrophil-induced injury of rat pulmonary alveolar epithelial cells. J Clin Invest. 1986 Nov;78(5):1375–1386. doi: 10.1172/JCI112724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Kotani M., Miyasaka M. Characterization of the rat leukocyte integrin, CD11/CD18, by the use of LFA-1 subunit-specific monoclonal antibodies. Eur J Immunol. 1991 Mar;21(3):627–633. doi: 10.1002/eji.1830210314. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Miyasaka M. Identification of monoclonal antibodies reactive with the rat homolog of ICAM-1, and evidence for a differential involvement of ICAM-1 in the adherence of resting versus activated lymphocytes to high endothelial cells. Int Immunol. 1990;2(2):165–171. doi: 10.1093/intimm/2.2.165. [DOI] [PubMed] [Google Scholar]
- Warren J. S. Intrapulmonary interleukin 1 mediates acute immune complex alveolitis in the rat. Biochem Biophys Res Commun. 1991 Mar 15;175(2):604–610. doi: 10.1016/0006-291x(91)91608-f. [DOI] [PubMed] [Google Scholar]
- Zimmerman G. A., Prescott S. M., McIntyre T. M. Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today. 1992 Mar;13(3):93–100. doi: 10.1016/0167-5699(92)90149-2. [DOI] [PubMed] [Google Scholar]