Abstract
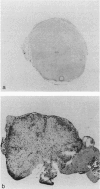
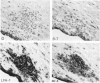
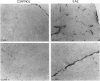
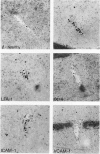
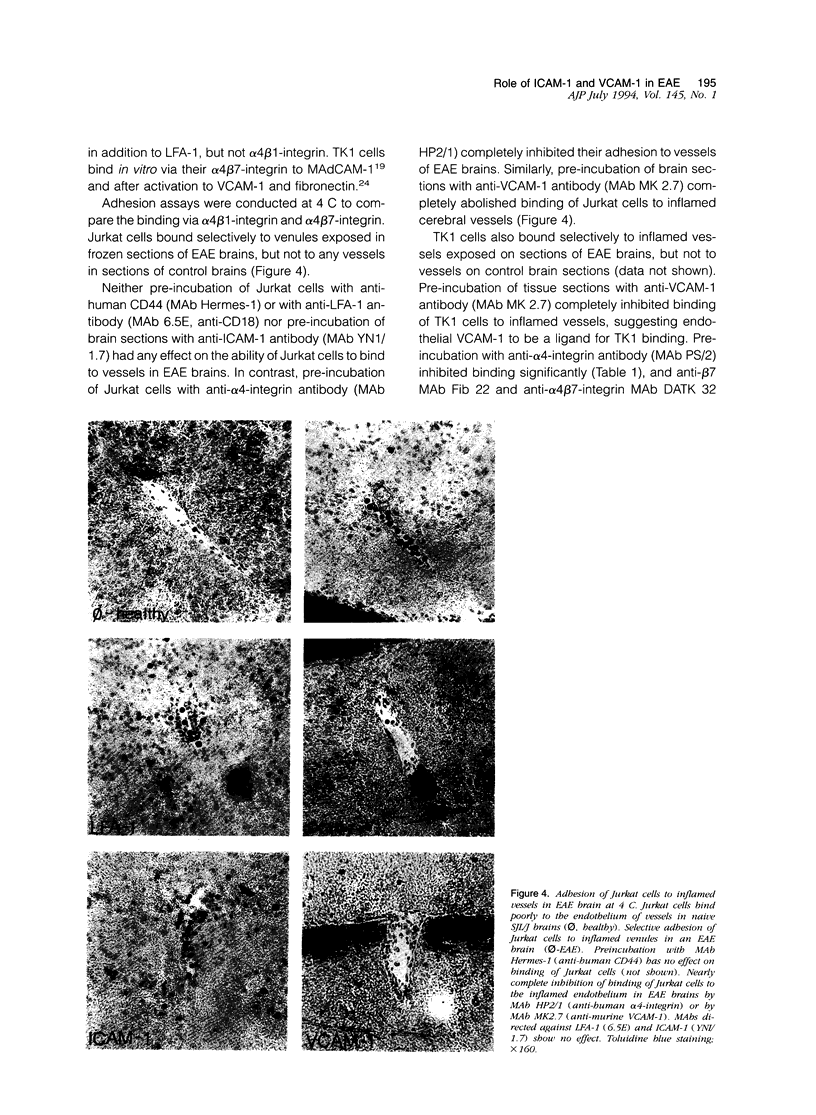
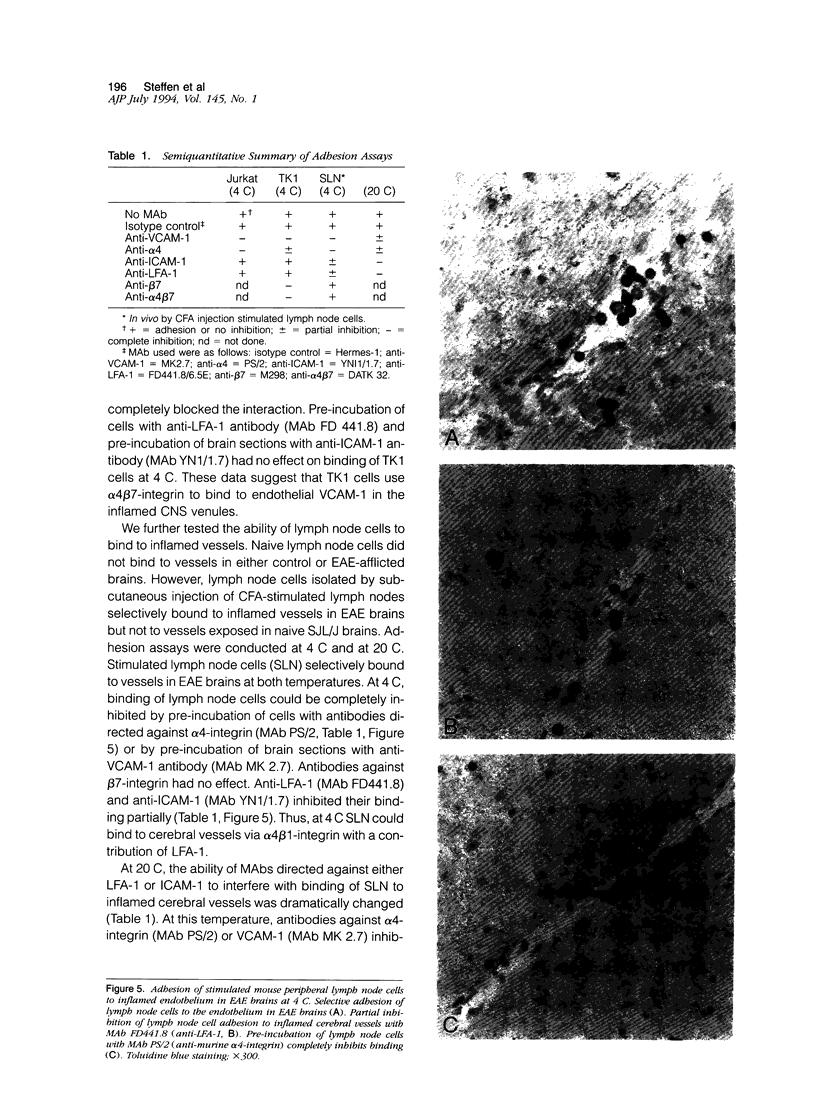
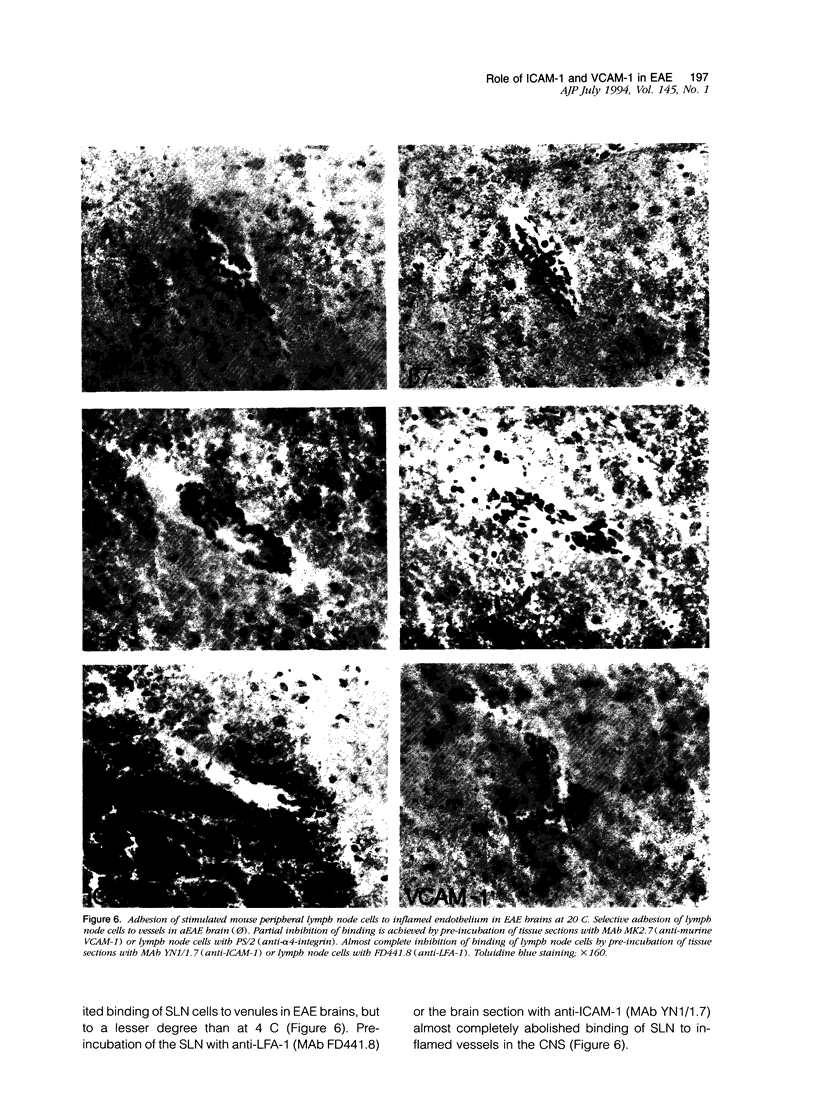
We have investigated the expression of vascular adhesion molecules during the first stage of chronic inflammation in experimental autoimmune encephalomyelitis in the SJL/J mouse. Immunocytochemical analysis of frozen sections of inflamed versus noninflamed brains and spinal cords showed that the vascular endothelium in brains and spinal cords from diseased animals expressed high levels of vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) but no detectable mucosal addressin or peripheral lymph node addressin. In frozen section assays, anti-alpha 4 integrin and anti-VCAM-1 monoclonal antibodies inhibited binding of mouse peripheral lymphocytes to inflamed brains at both 4 C and 20 C. Antilymphocyte function-associated antigen-1 and anti-ICAM-1 monoclonal antibodies inhibited binding of mouse peripheral lymphocytes to inflamed brains at 20 C. These results are consistent with an important role for the vascular adhesion molecules VCAM-1 and ICAM-1 and for their lymphocytes receptors in lymphocyte recruitment to the central nervous system.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archelos J. J., Jung S., Mäurer M., Schmied M., Lassmann H., Tamatani T., Miyasaka M., Toyka K. V., Hartung H. P. Inhibition of experimental autoimmune encephalomyelitis by an antibody to the intercellular adhesion molecule ICAM-1. Ann Neurol. 1993 Aug;34(2):145–154. doi: 10.1002/ana.410340209. [DOI] [PubMed] [Google Scholar]
- Bargatze R. F., Wu N. W., Weissman I. L., Butcher E. C. High endothelial venule binding as a predictor of the dissemination of passaged murine lymphomas. J Exp Med. 1987 Oct 1;166(4):1125–1131. doi: 10.1084/jem.166.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron J. L., Madri J. A., Ruddle N. H., Hashim G., Janeway C. A., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993 Jan 1;177(1):57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin C., Berg E. L., Briskin M. J., Andrew D. P., Kilshaw P. J., Holzmann B., Weissman I. L., Hamann A., Butcher E. C. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993 Jul 16;74(1):185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- Birkeland M. L., Metlay J., Sanders V. M., Fernandez-Botran R., Vitetta E. S., Steinman R. M., Puré E. Epitopes on CD45R [T200] molecules define differentiation antigens on murine B and T lymphocytes. J Mol Cell Immunol. 1988;4(2):71–85. [PubMed] [Google Scholar]
- Briskin M. J., McEvoy L. M., Butcher E. C. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature. 1993 Jun 3;363(6428):461–464. doi: 10.1038/363461a0. [DOI] [PubMed] [Google Scholar]
- Brown A. M., McFarlin D. E. Relapsing experimental allergic encephalomyelitis in the SJL/J mouse. Lab Invest. 1981 Sep;45(3):278–284. [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Campanero M. R., Pulido R., Ursa M. A., Rodríguez-Moya M., de Landázuri M. O., Sánchez-Madrid F. An alternative leukocyte homotypic adhesion mechanism, LFA-1/ICAM-1-independent, triggered through the human VLA-4 integrin. J Cell Biol. 1990 Jun;110(6):2157–2165. doi: 10.1083/jcb.110.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
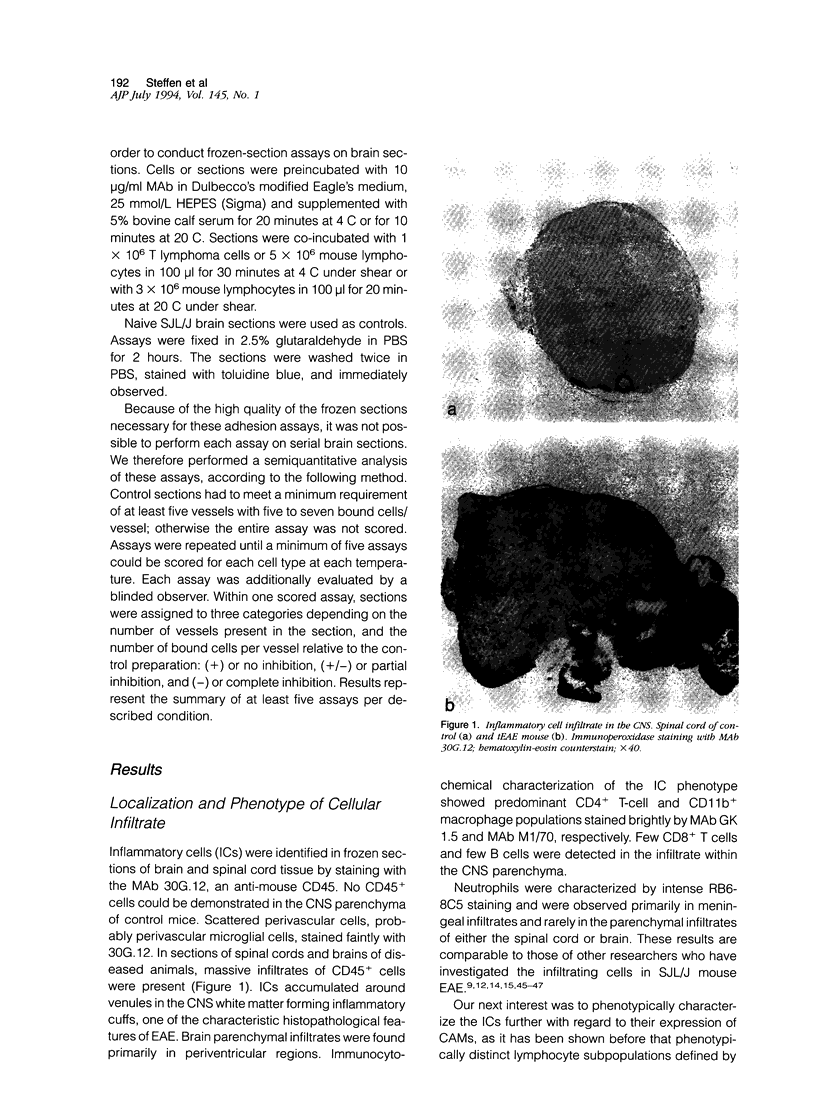
- Cannella B., Cross A. H., Raine C. S. Adhesion-related molecules in the central nervous system. Upregulation correlates with inflammatory cell influx during relapsing experimental autoimmune encephalomyelitis. Lab Invest. 1991 Jul;65(1):23–31. [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Coomber B. L., Stewart P. A. Morphometric analysis of CNS microvascular endothelium. Microvasc Res. 1985 Jul;30(1):99–115. doi: 10.1016/0026-2862(85)90042-1. [DOI] [PubMed] [Google Scholar]
- Crone C., Olesen S. P. Electrical resistance of brain microvascular endothelium. Brain Res. 1982 Jun 3;241(1):49–55. doi: 10.1016/0006-8993(82)91227-6. [DOI] [PubMed] [Google Scholar]
- Cross A. H., Cannella B., Brosnan C. F., Raine C. S. Homing to central nervous system vasculature by antigen-specific lymphocytes. I. Localization of 14C-labeled cells during acute, chronic, and relapsing experimental allergic encephalomyelitis. Lab Invest. 1990 Aug;63(2):162–170. [PubMed] [Google Scholar]
- Duijvestijn A. M., Kerkhove M., Bargatze R. F., Butcher E. C. Lymphoid tissue- and inflammation-specific endothelial cell differentiation defined by monoclonal antibodies. J Immunol. 1987 Feb 1;138(3):713–719. [PubMed] [Google Scholar]
- Fritz R. B., Chou C. H., McFarlin D. E. Induction of experimental allergic encephalomyelitis in PL/J and (SJL/J x PL/J)F1 mice by myelin basic protein and its peptides: localization of a second encephalitogenic determinant. J Immunol. 1983 Jan;130(1):191–194. [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Elices M. J., Parker C., Takada Y. Structure of the integrin VLA-4 and its cell-cell and cell-matrix adhesion functions. Immunol Rev. 1990 Apr;114:45–65. doi: 10.1111/j.1600-065x.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Holzmann B., McIntyre B. W., Weissman I. L. Identification of a murine Peyer's patch--specific lymphocyte homing receptor as an integrin molecule with an alpha chain homologous to human VLA-4 alpha. Cell. 1989 Jan 13;56(1):37–46. doi: 10.1016/0092-8674(89)90981-1. [DOI] [PubMed] [Google Scholar]
- Hänninen A., Taylor C., Streeter P. R., Stark L. S., Sarte J. M., Shizuru J. A., Simell O., Michie S. A. Vascular addressins are induced on islet vessels during insulitis in nonobese diabetic mice and are involved in lymphoid cell binding to islet endothelium. J Clin Invest. 1993 Nov;92(5):2509–2515. doi: 10.1172/JCI116859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof B. A., Ruiz P., Hesse B., Palacios R., Dunon D. EA-1, a novel adhesion molecule involved in the homing of progenitor T lymphocytes to the thymus. J Cell Biol. 1991 Sep;114(5):1069–1078. doi: 10.1083/jcb.114.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S. T., Bargatze R. F., Herron L. R., Butcher E. C. A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man. Eur J Immunol. 1986 Oct;16(10):1195–1202. doi: 10.1002/eji.1830161003. [DOI] [PubMed] [Google Scholar]
- Kilshaw P. J., Baker K. C. A unique surface antigen on intraepithelial lymphocytes in the mouse. Immunol Lett. 1988 Jun;18(2):149–154. doi: 10.1016/0165-2478(88)90056-9. [DOI] [PubMed] [Google Scholar]
- Kilshaw P. J., Murant S. J. Expression and regulation of beta 7(beta p) integrins on mouse lymphocytes: relevance to the mucosal immune system. Eur J Immunol. 1991 Oct;21(10):2591–2597. doi: 10.1002/eji.1830211041. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Berg E. L., Butcher E. C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989 Sep 15;245(4923):1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- LEE J. M., OLITSKY P. K. Simple method for enhancing development of acute disseminated encephalomyelitis in mice. Proc Soc Exp Biol Med. 1955 Jun;89(2):263–266. doi: 10.3181/00379727-89-21778. [DOI] [PubMed] [Google Scholar]
- Lee W. T., Vitetta E. S. The differential expression of homing and adhesion molecules on virgin and memory T cells in the mouse. Cell Immunol. 1991 Jan;132(1):215–222. doi: 10.1016/0008-8749(91)90020-c. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Marston W. L., Dudler L., Spertini O., Tedder T. F., Hein W. R. Tissue-specific migration pathways by phenotypically distinct subpopulations of memory T cells. Eur J Immunol. 1992 Apr;22(4):887–895. doi: 10.1002/eji.1830220402. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Miyake K., Medina K., Ishihara K., Kimoto M., Auerbach R., Kincade P. W. A VCAM-like adhesion molecule on murine bone marrow stromal cells mediates binding of lymphocyte precursors in culture. J Cell Biol. 1991 Aug;114(3):557–565. doi: 10.1083/jcb.114.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Weissman I. L., Greenberger J. S., Kincade P. W. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991 Mar 1;173(3):599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naparstek Y., Holoshitz J., Eisenstein S., Reshef T., Rappaport S., Chemke J., Ben-Nun A., Cohen I. R. Effector T lymphocyte line cells migrate to the thymus and persist there. Nature. 1982 Nov 18;300(5889):262–264. doi: 10.1038/300262a0. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990 Jul 13;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- Pettinelli C. B., McFarlin D. E. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2- T lymphocytes. J Immunol. 1981 Oct;127(4):1420–1423. [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Raine C. S., Barnett L. B., Brown A., Behar T., McFarlin D. E. Neuropathology of experimental allergic encephalomyelitis in inbred strains of mice. Lab Invest. 1980 Aug;43(2):150–157. [PubMed] [Google Scholar]
- Reese T. S., Karnovsky M. J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967 Jul;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlein R., Springer T. A. The requirement for lymphocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986 May 1;163(5):1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegg C., Postigo A. A., Sikorski E. E., Butcher E. C., Pytela R., Erle D. J. Role of integrin alpha 4 beta 7/alpha 4 beta P in lymphocyte adherence to fibronectin and VCAM-1 and in homotypic cell clustering. J Cell Biol. 1992 Apr;117(1):179–189. doi: 10.1083/jcb.117.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Gopal T. V., Horgan K. J., Graber N., Beall L. D., van Seventer G. A., Shaw S. Four molecular pathways of T cell adhesion to endothelial cells: roles of LFA-1, VCAM-1, and ELAM-1 and changes in pathway hierarchy under different activation conditions. J Cell Biol. 1991 Jun;113(5):1203–1212. doi: 10.1083/jcb.113.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram S., Solomon D., Rouse R. V., Steinman L. Identification of T cell subsets and B lymphocytes in mouse brain experimental allergic encephalitis lesions. J Immunol. 1982 Oct;129(4):1649–1651. [PubMed] [Google Scholar]
- Stamper H. B., Jr, Woodruff J. J. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976 Sep 1;144(3):828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter P. R., Rouse B. T., Butcher E. C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988 Nov;107(5):1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traugott U., Raine C. S., McFarlin D. E. Acute experimental allergic encephalomyelitis in the mouse: immunopathology of the developing lesion. Cell Immunol. 1985 Mar;91(1):240–254. doi: 10.1016/0008-8749(85)90047-4. [DOI] [PubMed] [Google Scholar]
- Trotter J., Steinman L. Homing of Lyt-2+ and Lyt-2- T cell subsets and B lymphocytes to the central nervous system of mice with acute experimental allergic encephalomyelitis. J Immunol. 1984 Jun;132(6):2919–2923. [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H., Sun D., Oropeza-Wekerle R. L., Meyermann R. Immune reactivity in the nervous system: modulation of T-lymphocyte activation by glial cells. J Exp Biol. 1987 Sep;132:43–57. doi: 10.1242/jeb.132.1.43. [DOI] [PubMed] [Google Scholar]
- Yednock T. A., Cannon C., Fritz L. C., Sanchez-Madrid F., Steinman L., Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992 Mar 5;356(6364):63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- Zeine R., Owens T. Direct demonstration of the infiltration of murine central nervous system by Pgp-1/CD44high CD45RB(low) CD4+ T cells that induce experimental allergic encephalomyelitis. J Neuroimmunol. 1992 Sep;40(1):57–69. doi: 10.1016/0165-5728(92)90213-5. [DOI] [PubMed] [Google Scholar]