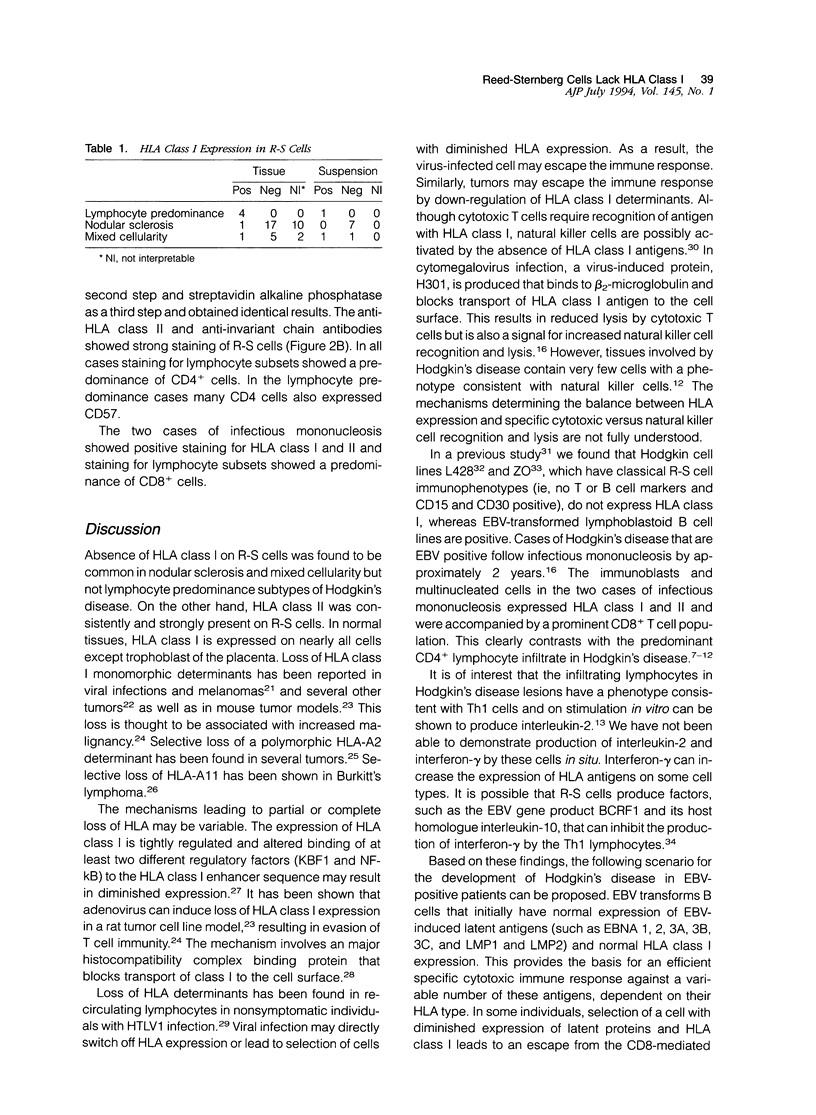
Abstract
The reactive cell population in Hodgkin's disease consists of predominantly CD4+ helper T cells and lacks CD8+ cytotoxic T cells and natural killer cells. This lack of a CD8+ response is surprising in view of the expression of the latent Epstein-Barr viral protein LMP by Reed-Sternberg cells in many cases of Hodgkin's disease, Deficient HLA class I expression would be one possible mechanism to avoid a CD8+ cytotoxic immune response. To test this possibility we studied the expression of HLA class I and II determinants on Reed-Sternberg cells in tissue sections and cell suspensions of Hodgkin's disease. Frozen tissue sections of 40 cases and cytocentrifuge preparations from cell suspensions of 10 lymph nodes involved by Hodgkin's disease were studied with monoclonal antibodies reactive with HLA determinants. As a control frozen tissue sections of two cases of infectious mononucleosis were studied. Careful examination of the tissue sections and subsequently of cytospins of cell suspensions showed that the Reed-Sternberg cells frequently lacked HLA class I but showed strong staining for HLA class II. Absence of HLA class I expression on Reed-Sternberg cells and their variants provides an explanation for the lack of a CD8+ cytotoxic immune response against antigens expressed on Reed-Sternberg cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbondanzo S. L., Sato N., Straus S. E., Jaffe E. S. Acute infectious mononucleosis. CD30 (Ki-1) antigen expression and histologic correlations. Am J Clin Pathol. 1990 May;93(5):698–702. doi: 10.1093/ajcp/93.5.698. [DOI] [PubMed] [Google Scholar]
- Aisenberg A. C., Wilkes B. M. Lymph node T cells in Hodgkin's disease: analysis of suspensions with monoclonal antibody and rosetting techniques. Blood. 1982 Mar;59(3):522–527. [PubMed] [Google Scholar]
- Andersson M., Päbo S., Nilsson T., Peterson P. A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985 Nov;43(1):215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Blanchet O., Bourge J. F., Zinszner H., Israel A., Kourilsky P., Dausset J., Degos L., Paul P. Altered binding of regulatory factors to HLA class I enhancer sequence in human tumor cell lines lacking class I antigen expression. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3488–3492. doi: 10.1073/pnas.89.8.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitz M. J., Croker B. P., Metzgar R. S. Immunohistochemical analysis of the distribution of lymphocyte subpopulations in Hodgkin's disease. Cancer Treat Rep. 1982 Apr;66(4):667–674. [PubMed] [Google Scholar]
- Dorreen M. S., Habeshaw J. A., Wrigley P. F., Lister T. A. Distribution of T-lymphocyte subsets in Hodgkin's disease characterized by monoclonal antibodies. Br J Cancer. 1982 Apr;45(4):491–499. doi: 10.1038/bjc.1982.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott B. E., Carlow D. A., Rodricks A. M., Wade A. Perspectives on the role of MHC antigens in normal and malignant cell development. Adv Cancer Res. 1989;53:181–245. doi: 10.1016/s0065-230x(08)60282-1. [DOI] [PubMed] [Google Scholar]
- Khanna R., Burrows S. R., Kurilla M. G., Jacob C. A., Misko I. S., Sculley T. B., Kieff E., Moss D. J. Localization of Epstein-Barr virus cytotoxic T cell epitopes using recombinant vaccinia: implications for vaccine development. J Exp Med. 1992 Jul 1;176(1):169–176. doi: 10.1084/jem.176.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H. G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Martin J. M., Warnke R. A. A quantitative comparison of T-cell subsets in Hodgkin's disease and reactive hyperplasia. Frozen section immunohistochemistry. Cancer. 1984 Jun 1;53(11):2450–2455. doi: 10.1002/1097-0142(19840601)53:11<2450::aid-cncr2820531115>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Masucci M. G., Torsteindottir S., Colombani J., Brautbar C., Klein E., Klein G. Down-regulation of class I HLA antigens and of the Epstein-Barr virus-encoded latent membrane protein in Burkitt lymphoma lines. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4567–4571. doi: 10.1073/pnas.84.13.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley D. J., Pound J. D. Modulation of MHC antigen expression by viruses and oncogenes. Immunol Today. 1991 Dec;12(12):429–431. doi: 10.1016/0167-5699(91)90013-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R. Role of a new cytokine, interleukin-10, in the cross-regulation of T helper cells. Ann N Y Acad Sci. 1991;628:337–344. doi: 10.1111/j.1749-6632.1991.tb17266.x. [DOI] [PubMed] [Google Scholar]
- Murray R. J., Kurilla M. G., Brooks J. M., Thomas W. A., Rowe M., Kieff E., Rickinson A. B. Identification of target antigens for the human cytotoxic T cell response to Epstein-Barr virus (EBV): implications for the immune control of EBV-positive malignancies. J Exp Med. 1992 Jul 1;176(1):157–168. doi: 10.1084/jem.176.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. J., Wang D., Young L. S., Wang F., Rowe M., Kieff E., Rickinson A. B. Epstein-Barr virus-specific cytotoxic T-cell recognition of transfectants expressing the virus-coded latent membrane protein LMP. J Virol. 1988 Oct;62(10):3747–3755. doi: 10.1128/jvi.62.10.3747-3755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali P. G., Nicotra M. R., Bigotti A., Venturo I., Marcenaro L., Giacomini P., Russo C. Selective changes in expression of HLA class I polymorphic determinants in human solid tumors. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6719–6723. doi: 10.1073/pnas.86.17.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppema S., Bhan A. K., Reinherz E. L., Posner M. R., Schlossman S. F. In situ immunologic characterization of cellular constituents in lymph nodes and spleens involved by Hodgkin's disease. Blood. 1982 Feb;59(2):226–232. [PubMed] [Google Scholar]
- Poppema S., Kaleta J., Hepperle B. Chromosomal abnormalities in patients with Hodgkin's disease: evidence for frequent involvement of the 14q chromosomal region but infrequent bcl-2 gene rearrangement in Reed-Sternberg cells. J Natl Cancer Inst. 1992 Dec 2;84(23):1789–1793. doi: 10.1093/jnci/84.23.1789. [DOI] [PubMed] [Google Scholar]
- Poppema S., Kaleta J., Hepperle B., Visser L. Biology of Hodgkin's disease. Ann Oncol. 1992 Sep;3 (Suppl 4):5–8. doi: 10.1093/annonc/3.suppl_4.s5. [DOI] [PubMed] [Google Scholar]
- Poppema S. The nature of the lymphocytes surrounding Reed-Sternberg cells in nodular lymphocyte predominance and in other types of Hodgkin's disease. Am J Pathol. 1989 Aug;135(2):351–357. [PMC free article] [PubMed] [Google Scholar]
- Poppema S., Visser L., de Jong B., Brinker M., Atmosoerodjo J., Timens W. The typical Reed-Sternberg phenotype and Ig gene rearrangement of Hodgkin's disease derived cell line ZO indicating a B-cell origin. Recent Results Cancer Res. 1989;117:67–74. doi: 10.1007/978-3-642-83781-4_7. [DOI] [PubMed] [Google Scholar]
- Poppema S., van Imhoff G., Torensma R., Smit J. Lymphadenopathy morphologically consistent with Hodgkin's disease associated with Epstein-Barr virus infection. Am J Clin Pathol. 1985 Sep;84(3):385–390. doi: 10.1093/ajcp/84.3.385. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Del Prete G. F., Maggi E., Bosi A., Bernardi F., Ponticelli P., Di Lollo S., Ricci M. Displacement of T lymphocytes with the 'Helper/Inducer' phenotype from peripheral blood to lymphoid organs in untreated patients with Hodgkin's disease. Scand J Haematol. 1983 Oct;31(4):305–314. doi: 10.1111/j.1600-0609.1983.tb00658.x. [DOI] [PubMed] [Google Scholar]
- Ruiter D. J., Bhan A. K., Harrist T. J., Sober A. J., Mihm M. C., Jr Major histocompatibility antigens and mononuclear inflammatory infiltrate in benign nevomelanocytic proliferations and malignant melanoma. J Immunol. 1982 Dec;129(6):2808–2815. [PubMed] [Google Scholar]
- Schaadt M., Diehl V., Stein H., Fonatsch C., Kirchner H. H. Two neoplastic cell lines with unique features derived from Hodgkin's disease. Int J Cancer. 1980 Dec 15;26(6):723–731. doi: 10.1002/ijc.2910260605. [DOI] [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Sonoda S., Yashiki S., Takahashi K., Arima N., Daitoku Y., Matsumoto M., Matsumoto T., Tara M., Shinmyozu K., Sato K. Altered HLA antigens expressed on T and B lymphocytes of adult T-cell leukemia/lymphoma patients and their relatives. Int J Cancer. 1987 Nov 15;40(5):629–634. doi: 10.1002/ijc.2910400510. [DOI] [PubMed] [Google Scholar]
- Staal S. P., Ambinder R., Beschorner W. E., Hayward G. S., Mann R. A survey of Epstein-Barr virus DNA in lymphoid tissue. Frequent detection in Hodgkin's disease. Am J Clin Pathol. 1989 Jan;91(1):1–5. doi: 10.1093/ajcp/91.1.1. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Strickler J. G., Warnke R. A., Purtilo D. T., Sklar J. Epstein-Barr viral DNA in tissues of Hodgkin's disease. Am J Pathol. 1987 Oct;129(1):86–91. [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Deacon E. M., Rowe M., Crocker J., Herbst H., Niedobitek G., Hamilton-Dutoit S. J., Pallesen G. Epstein-Barr virus latent genes in tumour cells of Hodgkin's disease. Lancet. 1991 Jun 29;337(8757):1617–1617. doi: 10.1016/0140-6736(91)93322-z. [DOI] [PubMed] [Google Scholar]




