Abstract
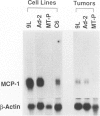
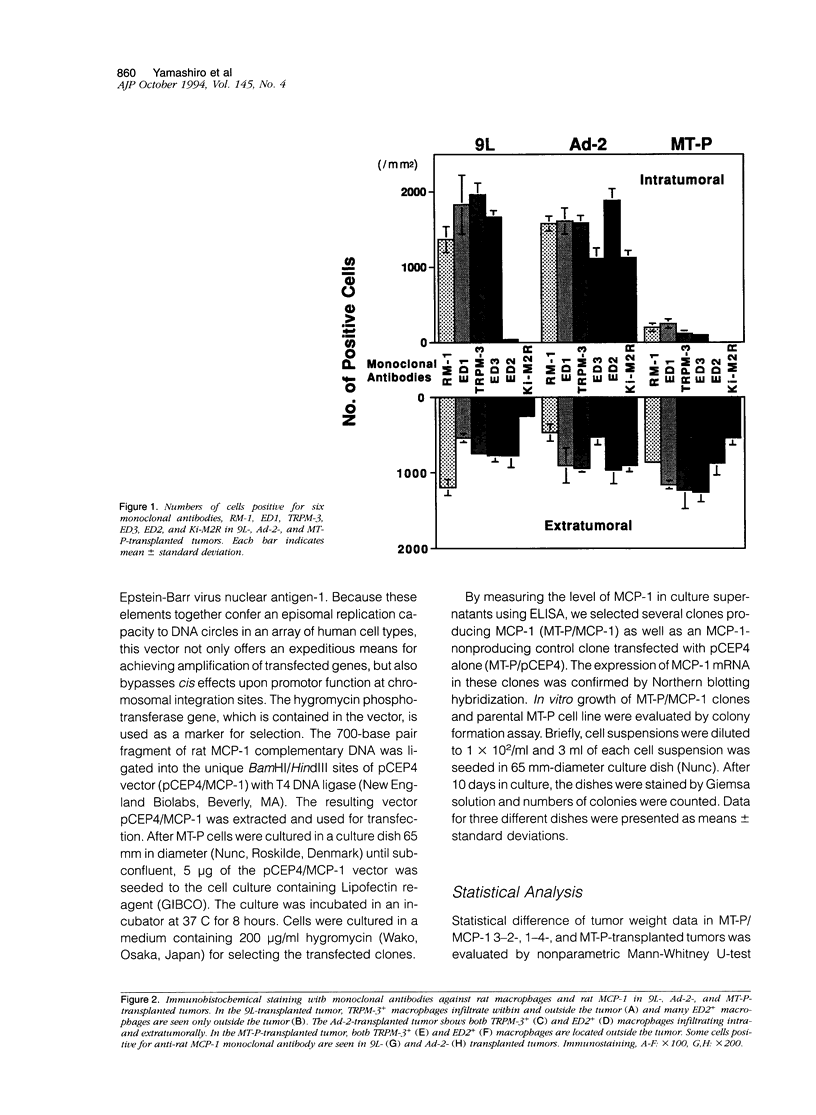
By immunohistochemistry using anti-rat macrophage monoclonal antibodies RM-1, ED1, ED2, ED3, TRPM-3, and Ki-M2R, we studied transplanted rat tumors of 9L (rat gliosarcoma), Ad-2 (rat mammary carcinoma), and MT-P (rat malignant fibrous histiocytoma) cell lines to examine the distribution pattern of macrophages within and around the tumors. Most tumor-associated macrophages expressed RM-1, ED1, and Ia antigens, indicating activated macrophages. Based on differences in their immunophenotypical expression, these macrophages were distinguished into two major subpopulations. One expressed TRPM-3 and/or ED3, and the other was positive for ED2 and Ki-M2R. The former was considered to be monocyte-derived macrophages, whereas the latter showed the immunophenotype of tissue-fixed, resident macrophages. Infiltration and distribution patterns in the two macrophage subpopulations differed in the three different tumors. Monocyte-derived, activated macrophages infiltrated into 9L- and Ad-2-transplanted tumors, which markedly produced monocyte chemoattractant protein-1 (MCP-1). Additionally, numerous ED2- and Ki-M2R-positive macrophages were observed within the Ad-2-transplanted tumors, and some of them expressed TRPM-3. However, there were few macrophages in the MT-P-transplanted tumors that showed no MCP-1 production. In transplanted tumors of four MT-P/MCP-1 cell lines established by transfecting a rat MCP-1 gene expression vector (pCEP4/MCP-1) into the MT-P cell line, different levels of MCP-1 production were detected, which correlated well with the numbers of intratumorally infiltrated TRPM-3-positive macrophages. In contrast, ED2- and Ki-M2R-positive macrophages were not detected in any MT-P/MCP-1-transplanted tumors. MT-P/MCP-1-transplanted tumors exhibited lower growth rate than parental MT-P-transplanted tumors. These results indicate that tumor-derived MCP-1 induces intratumoral infiltration of monocyte-derived macrophages, but not macrophages with the immunophenotype of tissue-fixed, resident type. The former population of macrophages seems to have a suppressive effect on the growth of tumors.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbé E., Damoiseaux J. G., Döpp E. A., Dijkstra C. D. Characterization and expression of the antigen present on resident rat macrophages recognized by monoclonal antibody ED2. Immunobiology. 1990 Dec;182(1):88–99. doi: 10.1016/S0171-2985(11)80586-3. [DOI] [PubMed] [Google Scholar]
- Barclay A. N. Different reticular elements in rat lymphoid tissue identified by localization of Ia, Thy-1 and MRC OX 2 antigens. Immunology. 1981 Dec;44(4):727–736. [PMC free article] [PubMed] [Google Scholar]
- Beelen R. H., Broekhuis-Fluitsma D. M., Korn C., Hoefsmit C. M. Identification of exudate-resident macrophages on the basis of peroxidatic activity. J Reticuloendothel Soc. 1978 Feb;23(2):103–110. [PubMed] [Google Scholar]
- Bottazzi B., Colotta F., Sica A., Nobili N., Mantovani A. A chemoattractant expressed in human sarcoma cells (tumor-derived chemotactic factor, TDCF) is identical to monocyte chemoattractant protein-1/monocyte chemotactic and activating factor (MCP-1/MCAF). Int J Cancer. 1990 Apr 15;45(4):795–797. doi: 10.1002/ijc.2910450436. [DOI] [PubMed] [Google Scholar]
- Bottazzi B., Polentarutti N., Acero R., Balsari A., Boraschi D., Ghezzi P., Salmona M., Mantovani A. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983 Apr 8;220(4593):210–212. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- Bottazzi B., Walter S., Govoni D., Colotta F., Mantovani A. Monocyte chemotactic cytokine gene transfer modulates macrophage infiltration, growth, and susceptibility to IL-2 therapy of a murine melanoma. J Immunol. 1992 Feb 15;148(4):1280–1285. [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Vogels I. M., Van Noorden C. J. Macrophages and dendritic cells in antigen-induced arthritis. An immunohistochemical study using cryostat sections of the whole knee joint of rat. Scand J Immunol. 1987 Nov;26(5):513–523. doi: 10.1111/j.1365-3083.1987.tb02285.x. [DOI] [PubMed] [Google Scholar]
- Graves D. T., Jiang Y. L., Williamson M. J., Valente A. J. Identification of monocyte chemotactic activity produced by malignant cells. Science. 1989 Sep 29;245(4925):1490–1493. doi: 10.1126/science.2781291. [DOI] [PubMed] [Google Scholar]
- Heuff G., van der Ende M. B., Boutkan H., Prevoo W., Bayon L. G., Fleuren G. J., Beelen R. H., Meijer S., Dijkstra C. D. Macrophage populations in different stages of induced hepatic metastases in rats: an immunohistochemical analysis. Scand J Immunol. 1993 Jul;38(1):10–16. doi: 10.1111/j.1365-3083.1993.tb01688.x. [DOI] [PubMed] [Google Scholar]
- Kuratsu J., Leonard E. J., Yoshimura T. Production and characterization of human glioma cell-derived monocyte chemotactic factor. J Natl Cancer Inst. 1989 Mar 1;81(5):347–351. doi: 10.1093/jnci/81.5.347. [DOI] [PubMed] [Google Scholar]
- Kuratsu J., Yoshizato K., Yoshimura T., Leonard E. J., Takeshima H., Ushio Y. Quantitative study of monocyte chemoattractant protein-1 (MCP-1) in cerebrospinal fluid and cyst fluid from patients with malignant glioma. J Natl Cancer Inst. 1993 Nov 17;85(22):1836–1839. doi: 10.1093/jnci/85.22.1836. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Bottazzi B., Colotta F., Sozzani S., Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992 Jul;13(7):265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- Ming W. J., Bersani L., Mantovani A. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J Immunol. 1987 Mar 1;138(5):1469–1474. [PubMed] [Google Scholar]
- Polman C. H., Dijkstra C. D., Sminia T., Koetsier J. C. Immunohistological analysis of macrophages in the central nervous system of Lewis rats with acute experimental allergic encephalomyelitis. J Neuroimmunol. 1986 May;11(3):215–222. doi: 10.1016/0165-5728(86)90005-6. [DOI] [PubMed] [Google Scholar]
- Schall T. J., Bacon K., Toy K. J., Goeddel D. V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990 Oct 18;347(6294):669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- Schmidek H. H., Nielsen S. L., Schiller A. L., Messer J. Morphological studies of rat brain tumors induced by N-nitrosomethylurea. J Neurosurg. 1971 Mar;34(3):335–340. doi: 10.3171/jns.1971.34.3.0335. [DOI] [PubMed] [Google Scholar]
- Takeya M., Hsiao L., Shimokawa Y., Takahashi K. Heterogeneity of rat macrophages recognized by monoclonal antibodies: an immunohistochemical and immunoelectron microscopic study. J Histochem Cytochem. 1989 May;37(5):635–641. doi: 10.1177/37.5.2649558. [DOI] [PubMed] [Google Scholar]
- Takeya M., Hsiao L., Takahashi K. A new monoclonal antibody, TRPM-3, binds specifically to certain rat macrophage populations: immunohistochemical and immunoelectron microscopic analysis. J Leukoc Biol. 1987 Mar;41(3):187–195. doi: 10.1002/jlb.41.3.187. [DOI] [PubMed] [Google Scholar]
- Verschure P. J., Van Noorden C. J., Dijkstra C. D. Macrophages and dendritic cells during the early stages of antigen-induced arthritis in rats: immunohistochemical analysis of cryostat sections of the whole knee joint. Scand J Immunol. 1989 Mar;29(3):371–381. doi: 10.1111/j.1365-3083.1989.tb01136.x. [DOI] [PubMed] [Google Scholar]
- Wacker H. H., Radzun H. J., Parwaresch M. R. Ki-M2R, a new specific monoclonal antibody, discriminates tissue macrophages from reticulum cells and monocytes in vivo and in vitro. J Leukoc Biol. 1985 Oct;38(4):509–520. doi: 10.1002/jlb.38.4.509. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S., Bottazzi B., Govoni D., Colotta F., Mantovani A. Macrophage infiltration and growth of sarcoma clones expressing different amounts of monocyte chemotactic protein/JE. Int J Cancer. 1991 Sep 30;49(3):431–435. doi: 10.1002/ijc.2910490321. [DOI] [PubMed] [Google Scholar]
- Wang J. M., Colella S., Allavena P., Mantovani A. Chemotactic activity of human recombinant granulocyte-macrophage colony-stimulating factor. Immunology. 1987 Mar;60(3):439–444. [PMC free article] [PubMed] [Google Scholar]
- Wang J. M., Griffin J. D., Rambaldi A., Chen Z. G., Mantovani A. Induction of monocyte migration by recombinant macrophage colony-stimulating factor. J Immunol. 1988 Jul 15;141(2):575–579. [PubMed] [Google Scholar]
- Yamate J., Tajima M., Togo M., Shibuya N., Shibuya K., Kudow S. Characteristics of in vitro passaged cells derived from a rat transplantable malignant fibrous histiocytoma. Nihon Juigaku Zasshi. 1989 Oct;51(5):861–869. doi: 10.1292/jvms1939.51.861. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Leonard E. J. Human monocyte chemoattractant protein-1: structure and function. Cytokines. 1992;4:131–152. [PubMed] [Google Scholar]
- Yoshimura T., Robinson E. A., Tanaka S., Appella E., Kuratsu J., Leonard E. J. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989 Apr 1;169(4):1449–1459. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Takeya M., Takahashi K., Kuratsu J., Leonard E. J. Production and characterization of mouse monoclonal antibodies against human monocyte chemoattractant protein-1. J Immunol. 1991 Oct 1;147(7):2229–2233. [PubMed] [Google Scholar]
- Yoshimura T., Takeya M., Takahashi K. Molecular cloning of rat monocyte chemoattractant protein-1 (MCP-1) and its expression in rat spleen cells and tumor cell lines. Biochem Biophys Res Commun. 1991 Jan 31;174(2):504–509. doi: 10.1016/0006-291x(91)91445-i. [DOI] [PubMed] [Google Scholar]