Abstract
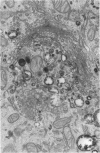
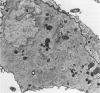
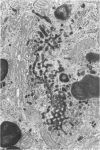
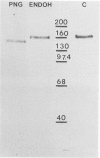
The importance of the Golgi apparatus in the transport, processing, and targeting of proteins destined for secretion, plasma membranes, and lysosomes has emerged from numerous studies. In this paper we review studies from our laboratory dealing with 1) the Golgi apparatus during mitosis and the role of microtubules in maintaining the structure of the organelle, 2) the endocytosis of antibodies, exogenous lectins, and toxins into the Golgi apparatus of several cells including neurons in vivo and in vitro, 3) the traffic of MG-160, a membrane sialoglycoprotein of the medial cisternae of the Golgi apparatus, from the trans-Golgi network to the Golgi cisternae, and 4) the involvement of the Golgi apparatus of motor neurons in the pathogenesis of amyotrophic lateral sclerosis. We conclude with a summary of ongoing work on the primary structure of MG-160 and introduce evidence suggesting that this intrinsic membrane protein of the Golgi apparatus may be involved in the regulation of endogenous, autocrine, basic fibroblast growth factor. We hope that this review will stimulate studies on the Golgi apparatus of neurons, which may lead to the discovery of neuron-specific properties of this important organelle and its involvement in the pathogenesis of neurodegenerative disorders.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara J. F., Cheng S. H., Smith A. E. Intracellular protein trafficking defects in human disease. Trends Cell Biol. 1992 May;2(5):145–149. doi: 10.1016/0962-8924(92)90101-r. [DOI] [PubMed] [Google Scholar]
- Antoine J. C., Avrameas S., Gonatas N. K., Stieber A., Gonatas J. O. Plasma membrane and internalized immunoglobulins of lymph node cells studied with conjugates of antibody or its Fab fragments with horseradish peroxidase. J Cell Biol. 1974 Oct;63(1):12–23. doi: 10.1083/jcb.63.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Peroxidase labelled antibody and Fab conjugates with enhanced intracellular penetration. Immunochemistry. 1971 Dec;8(12):1175–1179. doi: 10.1016/0019-2791(71)90395-8. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Barasch J., Al-Awqati Q. Chloride channels, Golgi pH and cystic fibrosis. Trends Cell Biol. 1992 Feb;2(2):35–37. doi: 10.1016/0962-8924(92)90149-h. [DOI] [PubMed] [Google Scholar]
- Burchanowski B. J., Hogue-Angeletti R., Stieber A., Gonatas J., Gonatas N. K. In 'undifferentiated' PC12 cells, GERL is not segregated from the Golgi apparatus. J Neurocytol. 1982 Apr;11(2):323–333. doi: 10.1007/BF01258249. [DOI] [PubMed] [Google Scholar]
- Burrus L. W., Zuber M. E., Lueddecke B. A., Olwin B. B. Identification of a cysteine-rich receptor for fibroblast growth factors. Mol Cell Biol. 1992 Dec;12(12):5600–5609. doi: 10.1128/mcb.12.12.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croul S. E., Mezitis S. G., Gonatas N. K. An anti-organelle antibody in pathology. The chromatolytic reaction studied with a monoclonal antibody against the Golgi apparatus. Am J Pathol. 1988 Nov;133(2):355–362. [PMC free article] [PubMed] [Google Scholar]
- Croul S., Mezitis S. G., Stieber A., Chen Y. J., Gonatas J. O., Goud B., Gonatas N. K. Immunocytochemical visualization of the Golgi apparatus in several species, including human, and tissues with an antiserum against MG-160, a sialoglycoprotein of rat Golgi apparatus. J Histochem Cytochem. 1990 Jul;38(7):957–963. doi: 10.1177/38.7.2355176. [DOI] [PubMed] [Google Scholar]
- Côté F., Collard J. F., Julien J. P. Progressive neuronopathy in transgenic mice expressing the human neurofilament heavy gene: a mouse model of amyotrophic lateral sclerosis. Cell. 1993 Apr 9;73(1):35–46. doi: 10.1016/0092-8674(93)90158-m. [DOI] [PubMed] [Google Scholar]
- Deng H. X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W. Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993 Aug 20;261(5124):1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas J. O., Gonatas N. K., Stieber A., Fleischer B. Isolation and characterization of an enriched Golgi fraction from neurons of developing rat brains. J Neurochem. 1985 Aug;45(2):497–507. doi: 10.1111/j.1471-4159.1985.tb04016.x. [DOI] [PubMed] [Google Scholar]
- Gonatas J. O., Gonatas N. K., Stieber A., Louvard D. Polypeptides of the Golgi apparatus of neurons from rat brain. J Neurochem. 1987 Nov;49(5):1498–1506. doi: 10.1111/j.1471-4159.1987.tb01020.x. [DOI] [PubMed] [Google Scholar]
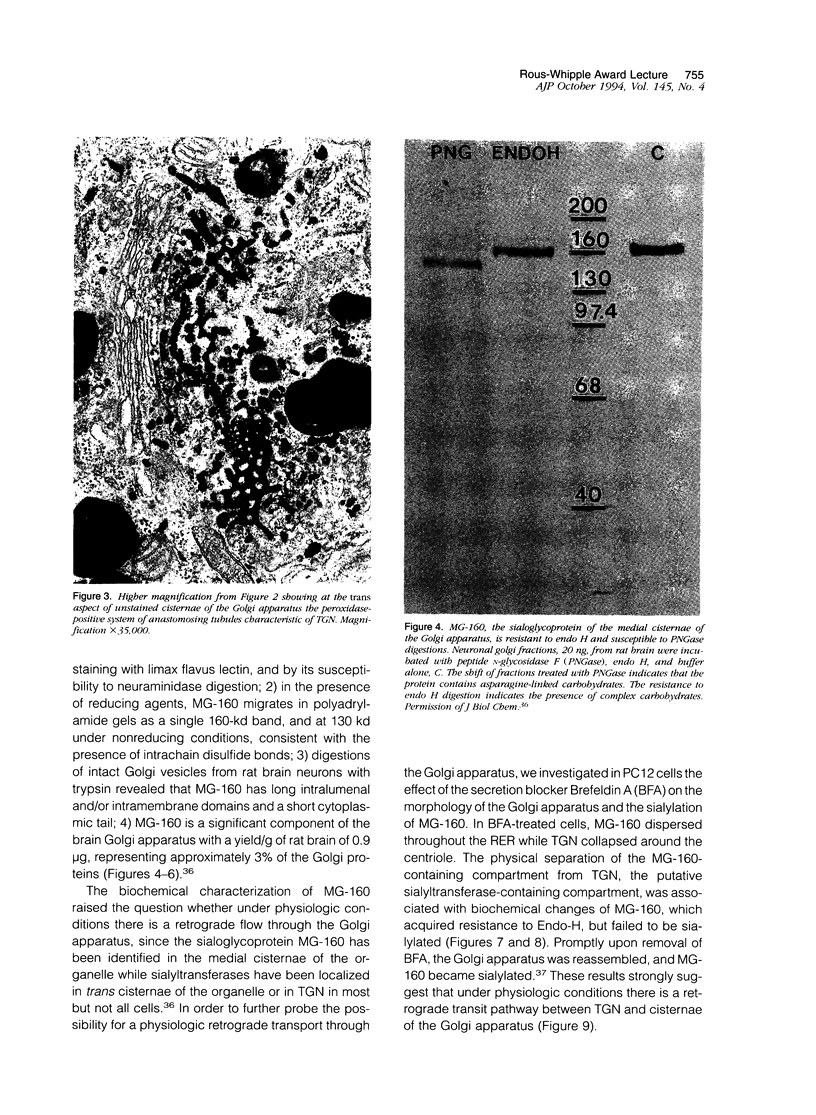
- Gonatas J. O., Mezitis S. G., Stieber A., Fleischer B., Gonatas N. K. MG-160. A novel sialoglycoprotein of the medial cisternae of the Golgi apparatus [published eeratum appears in J Biol Chem 1989 Mar 5;264(7):4264]. J Biol Chem. 1989 Jan 5;264(1):646–653. [PubMed] [Google Scholar]
- Gonatas J., Stieber A., Olsnes S., Gonatas N. K. Pathways involved in fluid phase and adsorptive endocytosis in neuroblastoma. J Cell Biol. 1980 Dec;87(3 Pt 1):579–588. doi: 10.1083/jcb.87.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas N. K., Avrameas S. Detection of plasma membrane carbohydrates with lectin peroxidase conjugates. J Cell Biol. 1973 Nov;59(2 Pt 1):436–443. doi: 10.1083/jcb.59.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas N. K., Gonatas J. O., Stieber A., Antoine J. C., Avrameas S. Quantitative ultrastructural autoradiographic studies of iodinated plasma membranes of lymphocytes during segregation and internalization of surface immunoglobulins. J Cell Biol. 1976 Sep;70(3):477–493. doi: 10.1083/jcb.70.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas N. K., Harper C., Mizutani T., Gonatas J. O. Superior sensitivity of conjugates of horseradish peroxidase with wheat germ agglutinin for studies of retrograde axonal transport. J Histochem Cytochem. 1979 Mar;27(3):728–734. doi: 10.1177/27.3.90065. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Kim S. U., Stieber A., Avrameas S. Internalization of lectins in neuronal GERL. J Cell Biol. 1977 Apr;73(1):1–13. doi: 10.1083/jcb.73.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas N. K. Presidential address. The role of neuronal golgi apparatus in a centripetal membrane vesicular traffic. J Neuropathol Exp Neurol. 1982 Jan;41(1):6–17. doi: 10.1097/00005072-198201000-00002. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Steiber A., Kim S. U., Graham D. I., Avrameas S. Internalization of neuronal plasma membrane ricin receptors into the Golgi apparatus. Exp Cell Res. 1975 Sep;94(2):426–431. doi: 10.1016/0014-4827(75)90508-x. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Stieber A., Gonatas J., Mommoi T., Fishman P. H. Endocytosis of exogenous GM1 ganglioside and cholera toxin by neuroblastoma cells. Mol Cell Biol. 1983 Jan;3(1):91–101. doi: 10.1128/mcb.3.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas N. K., Stieber A., Hickey W. F., Herbert S. H., Gonatas J. O. Endosomes and Golgi vesicles in adsorptive and fluid phase endocytosis. J Cell Biol. 1984 Oct;99(4 Pt 1):1379–1390. doi: 10.1083/jcb.99.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas N. K., Stieber A., Mourelatos Z., Chen Y., Gonatas J. O., Appel S. H., Hays A. P., Hickey W. F., Hauw J. J. Fragmentation of the Golgi apparatus of motor neurons in amyotrophic lateral sclerosis. Am J Pathol. 1992 Mar;140(3):731–737. [PMC free article] [PubMed] [Google Scholar]
- Goud B., Antoine J. C., Gonatas N. K., Stieber A., Avrameas S. A comparative study of fluid-phase and adsorptive endocytosis of horseradish peroxidase in lymphoid cells. Exp Cell Res. 1981 Apr;132(2):375–386. doi: 10.1016/0014-4827(81)90113-0. [DOI] [PubMed] [Google Scholar]
- Goud B., Antoine J. C., Gonatas N. K., Stieber A., Avrameas S. A morphological and functional study on antigen binding and endocytosis by immunocytes. Immunology. 1980 Dec;41(4):833–848. [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Harper C. G., Gonatas J. O., Mizutani T., Gonatas N. K. Retrograde transport and effects of toxic ricin in the autonomic nervous system. Lab Invest. 1980 Apr;42(4):396–404. [PubMed] [Google Scholar]
- Harper C. G., Gonatas J. O., Stieber A., Gonatas N. K. In vivo uptake of wheat germ agglutinin-horseradish peroxidase conjugates into neuronal GERL and lysosomes. Brain Res. 1980 Apr 28;188(2):465–472. doi: 10.1016/0006-8993(80)90045-1. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Stieber A., Hogue-Angeletti R., Gonatas J., GOnatas N. K. Nerve growth factor induced changes in the Golgi apparatus of PC-12 rat pheochromocytoma cells as studied by ligand endocytosis, cytochemical and morphometric methods. J Neurocytol. 1983 Oct;12(5):751–766. doi: 10.1007/BF01258149. [DOI] [PubMed] [Google Scholar]
- Ho W. C., Allan V. J., van Meer G., Berger E. G., Kreis T. E. Reclustering of scattered Golgi elements occurs along microtubules. Eur J Cell Biol. 1989 Apr;48(2):250–263. [PubMed] [Google Scholar]
- Joseph K. C., Kim S. U., Stieber A., Gonatas N. K. Endocytosis of cholera toxin into neuronal GERL. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2815–2819. doi: 10.1073/pnas.75.6.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph K. C., Stieber A., Gonatas N. K. Endocytosis of cholera toxin in GERL-like structures of murine neuroblastoma cells pretreated with GM1 ganglioside. Cholera toxin internalization into Neuroblastoma GERL. J Cell Biol. 1979 Jun;81(3):543–554. doi: 10.1083/jcb.81.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karecla P. I., Kreis T. E. Interaction of membranes of the Golgi complex with microtubules in vitro. Eur J Cell Biol. 1992 Apr;57(2):139–146. [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992 Mar;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes. FASEB J. 1987 Dec;1(6):462–468. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]
- Kreis T. E. Role of microtubules in the organisation of the Golgi apparatus. Cell Motil Cytoskeleton. 1990;15(2):67–70. doi: 10.1002/cm.970150202. [DOI] [PubMed] [Google Scholar]
- Lencer W. I., de Almeida J. B., Moe S., Stow J. L., Ausiello D. A., Madara J. L. Entry of cholera toxin into polarized human intestinal epithelial cells. Identification of an early brefeldin A sensitive event required for A1-peptide generation. J Clin Invest. 1993 Dec;92(6):2941–2951. doi: 10.1172/JCI116917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucocq J. M., Pryde J. G., Berger E. G., Warren G. A mitotic form of the Golgi apparatus in HeLa cells. J Cell Biol. 1987 Apr;104(4):865–874. doi: 10.1083/jcb.104.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E. Targeting and retention of Golgi membrane proteins. Curr Opin Cell Biol. 1993 Aug;5(4):606–612. doi: 10.1016/0955-0674(93)90129-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara J. O., Fridovich I. Human genetics. Did radicals strike Lou Gehrig? Nature. 1993 Mar 4;362(6415):20–21. doi: 10.1038/362020a0. [DOI] [PubMed] [Google Scholar]
- Mellman I., Simons K. The Golgi complex: in vitro veritas? Cell. 1992 Mar 6;68(5):829–840. doi: 10.1016/0092-8674(92)90027-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezitis S. G., Stieber A., Gonatas N. K. Quantitative ultrastructural, autoradiographic evidence for the magnitude and early involvement of the Golgi apparatus complex in the endocytosis of wheat germ agglutinin by cultured neuroblastoma. J Cell Physiol. 1987 Sep;132(3):401–414. doi: 10.1002/jcp.1041320303. [DOI] [PubMed] [Google Scholar]
- Mourelatos Z., Adler H., Hirano A., Donnenfeld H., Gonatas J. O., Gonatas N. K. Fragmentation of the Golgi apparatus of motor neurons in amyotrophic lateral sclerosis revealed by organelle-specific antibodies. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4393–4395. doi: 10.1073/pnas.87.11.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourelatos Z., Yachnis A., Rorke L., Mikol J., Gonatas N. K. The Golgi apparatus of motor neurons in amyotrophic lateral sclerosis. Ann Neurol. 1993 Jun;33(6):608–615. doi: 10.1002/ana.410330609. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M. Cytochemical contributions to differentiating GERL from the Golgi apparatus. Histochem J. 1977 Sep;9(5):525–551. doi: 10.1007/BF01002901. [DOI] [PubMed] [Google Scholar]
- Pypaert M., Nilsson T., Berger E. G., Warren G. Mitotic Golgi clusters are not tubular endosomes. J Cell Sci. 1993 Mar;104(Pt 3):811–818. doi: 10.1242/jcs.104.3.811. [DOI] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. HISTOCHEMICAL AND ULTRASTRUCTURAL STUDIES ON HELA CELL CULTURES EXPOSED TO SPINDLE INHIBITORS WITH SPECIAL REFERENCE TO THE INTERPHASE CELL. J Histochem Cytochem. 1964 Sep;12:704–711. doi: 10.1177/12.9.704. [DOI] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. THE ULTRASTRUCTURE OF A MAMMALIAN CELL DURING THE MITOTIC CYCLE. J Cell Biol. 1964 Jun;21:429–463. doi: 10.1083/jcb.21.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes C. H., Stieber A., Gonatas N. K. A quantitative electron microscopic study of the intracellular localization of wheat germ agglutinin in retinal neurons. J Comp Neurol. 1986 Dec 15;254(3):287–296. doi: 10.1002/cne.902540303. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Garred O., Prydz K., Kozlov J. V., Hansen S. H., van Deurs B. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature. 1992 Aug 6;358(6386):510–512. doi: 10.1038/358510a0. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Prydz K., Hansen S. H., van Deurs B. Ricin transport in brefeldin A-treated cells: correlation between Golgi structure and toxic effect. J Cell Biol. 1991 Nov;115(4):971–981. doi: 10.1083/jcb.115.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenenberger C. A., Zuk A., Zinkl G. M., Kendall D., Matlin K. S. Integrin expression and localization in normal MDCK cells and transformed MDCK cells lacking apical polarity. J Cell Sci. 1994 Feb;107(Pt 2):527–541. doi: 10.1242/jcs.107.2.527. [DOI] [PubMed] [Google Scholar]
- Stieber A., Gonatas J. O., Gonatas N. K. Differences between the endocytosis of horseradish peroxidase and its conjugate with wheat germ agglutinin by cultured fibroblasts. J Cell Physiol. 1984 Apr;119(1):71–76. doi: 10.1002/jcp.1041190112. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Gonatas J. O., Gonatas N. K. A light and electron microscopic study of the intraneuronal transport of horseradish peroxidase and wheat germ agglutinin-peroxidase conjugates in the rat visual system. J Neurocytol. 1981 Jun;10(3):441–456. doi: 10.1007/BF01262415. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Gonatas N. K. A morphometric study of the endocytosis of wheat germ agglutinin-horseradish peroxidase conjugates by retinal ganglion cells in the rat. Brain Res. 1983 Aug 8;272(2):201–210. doi: 10.1016/0006-8993(83)90566-8. [DOI] [PubMed] [Google Scholar]
- Xu Z., Cork L. C., Griffin J. W., Cleveland D. W. Increased expression of neurofilament subunit NF-L produces morphological alterations that resemble the pathology of human motor neuron disease. Cell. 1993 Apr 9;73(1):23–33. doi: 10.1016/0092-8674(93)90157-l. [DOI] [PubMed] [Google Scholar]