Abstract
The effects of smoke inhalation on alveolar surfactant subtypes were examined in mice exposed for 30 minutes to smoke generated from the burning of a flexible polyurethane foam. At 4 or 12 hours after the exposure, three surfactant pellets, P10, P60, and P100, and a supernatant, S100, were prepared by sequential centrifugation of lavage fluids at 10,000 g for 30 minutes (P10), 60,000 g for 60 minutes (P60), and 100,000 g for 15 hours (P100 and S100). Phospholipid analysis and electron microscopy were performed on each fraction. Smoke exposure dramatically altered the normal distributions of these fractions: it significantly increased the phospholipid content of the heavier subtype, P10, which is thought to represent newly secreted surfactant; had no effect on the intermediate form, P60; and dramatically increased the phospholipid content (approximately fivefold) of the lighter subtypes, P100 and S100, which are believed to represent catabolic end-products of alveolar surfactant. Only P100 was structurally altered by the smoke. These results represent alterations of the normal metabolic processing of alveolar surfactant. Whereas the mechanism is yet to be defined, it seems to involve a small but significant increase in the newly secreted surfactant, as well as an excessively high accumulation of the structurally altered catabolic forms of the secreted surfactant.
Full text
PDF

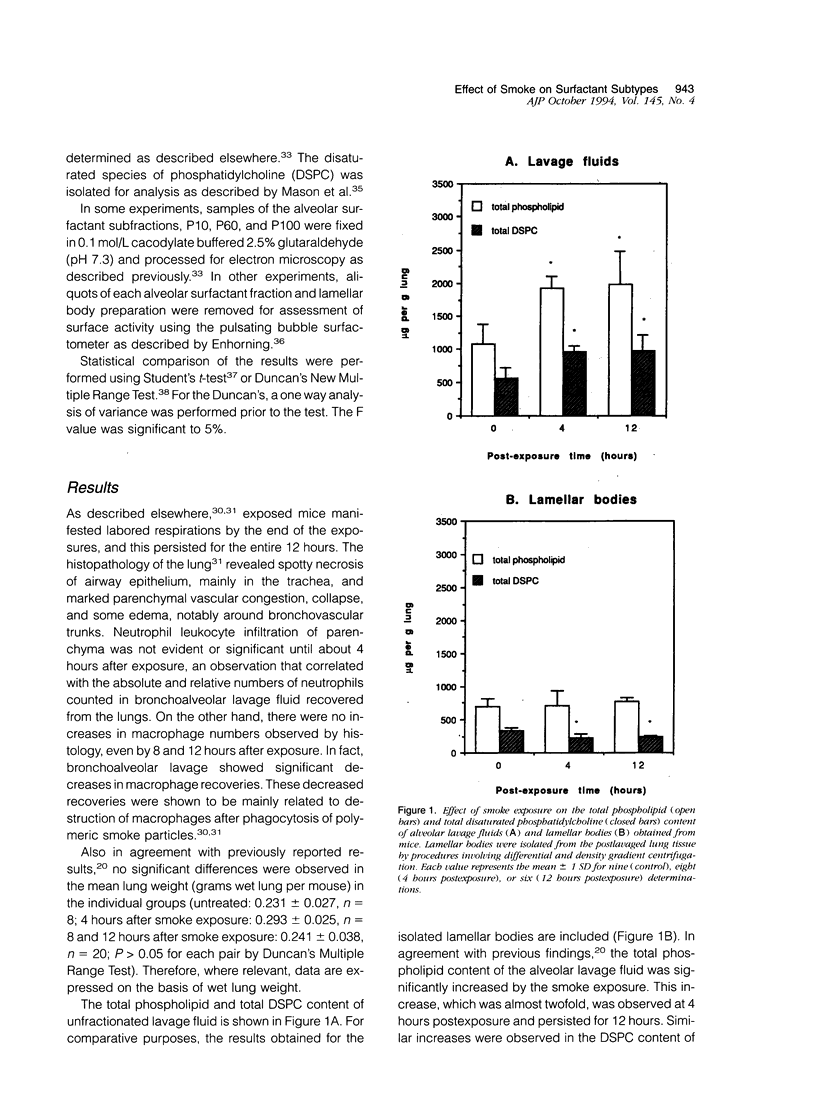
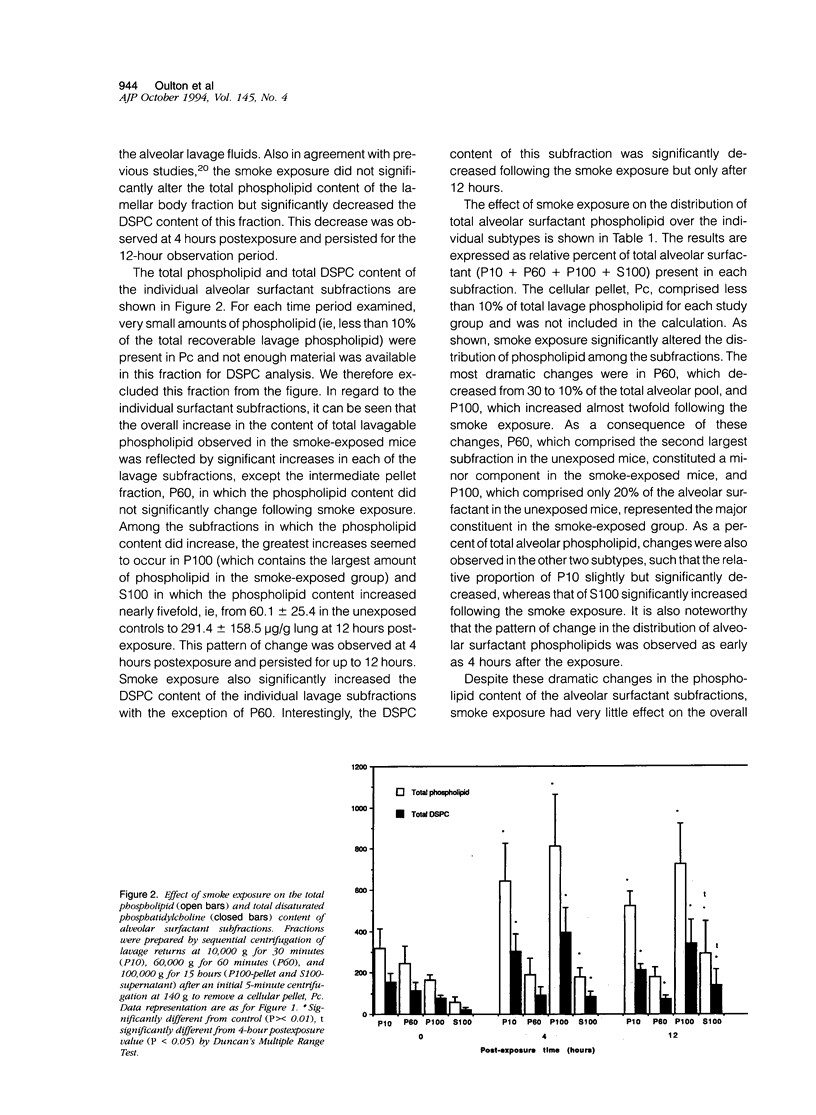
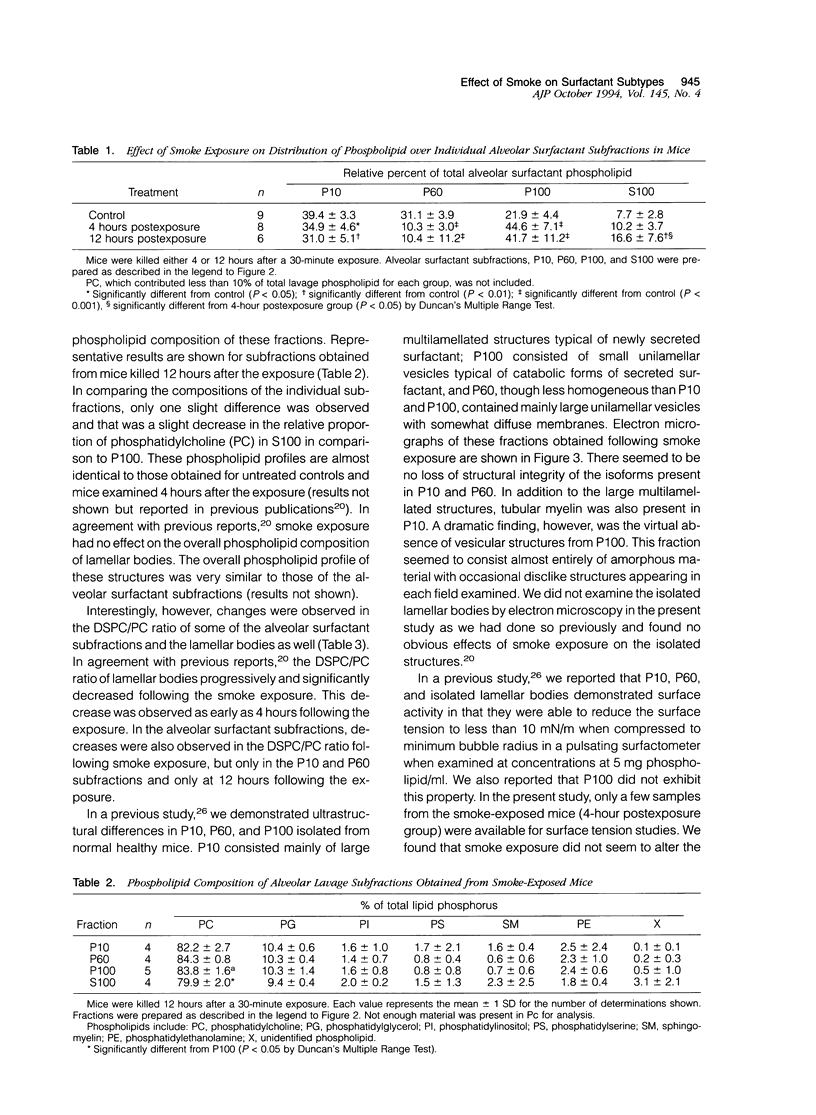





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amanuma K., Suzuki K. T. Effect of intratracheal instillation of cadmium chloride on phospholipids in alveolar wash fluid. Toxicology. 1987 Jun;44(3):321–328. doi: 10.1016/0300-483x(87)90033-3. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Balis J. U., Paterson J. F., Haller E. M., Shelley S. A., Montgomery M. R. Ozone-induced lamellar body responses in a rat model for alveolar injury and repair. Am J Pathol. 1988 Aug;132(2):330–344. [PMC free article] [PubMed] [Google Scholar]
- Balis J. U., Paterson J. F., Lundh J. M., Haller E. M., Shelley S. A., Montgomery M. R. Ozone stress initiates acute perturbations of secreted surfactant membranes. Am J Pathol. 1991 Apr;138(4):847–857. [PMC free article] [PubMed] [Google Scholar]
- Cockshutt A. M., Absolom D. R., Possmayer F. The role of palmitic acid in pulmonary surfactant: enhancement of surface activity and prevention of inhibition by blood proteins. Biochim Biophys Acta. 1991 Sep 11;1085(2):248–256. doi: 10.1016/0005-2760(91)90101-m. [DOI] [PubMed] [Google Scholar]
- Demling R. H. Burns. N Engl J Med. 1985 Nov 28;313(22):1389–1398. doi: 10.1056/NEJM198511283132205. [DOI] [PubMed] [Google Scholar]
- Dethloff L. A., Gilmore L. B., Hook G. E. The relationship between intra- and extra-cellular surfactant phospholipids in the lungs of rabbits and the effects of silica-induced lung injury. Biochem J. 1986 Oct 1;239(1):59–67. doi: 10.1042/bj2390059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs L. G. Pulmonary surfactant. Annu Rev Med. 1989;40:431–446. doi: 10.1146/annurev.me.40.020189.002243. [DOI] [PubMed] [Google Scholar]
- Enhorning G. Pulsating bubble technique for evaluating pulmonary surfactant. J Appl Physiol Respir Environ Exerc Physiol. 1977 Aug;43(2):198–203. doi: 10.1152/jappl.1977.43.2.198. [DOI] [PubMed] [Google Scholar]
- Eskelson C. D., Chvapil M., Strom K. A., Vostal J. J. Pulmonary phospholipidosis in rats respiring air containing diesel particulates. Environ Res. 1987 Dec;44(2):260–271. doi: 10.1016/s0013-9351(87)80235-9. [DOI] [PubMed] [Google Scholar]
- Giri S. N. Effects of intratracheal instillation of bleomycin on phospholipid synthesis in hamster lung tissue slices. Proc Soc Exp Biol Med. 1987 Dec;186(3):327–332. doi: 10.3181/00379727-186-42621. [DOI] [PubMed] [Google Scholar]
- Gross N. J., Narine K. R. Surfactant subtypes in mice: characterization and quantitation. J Appl Physiol (1985) 1989 Jan;66(1):342–349. doi: 10.1152/jappl.1989.66.1.342. [DOI] [PubMed] [Google Scholar]
- Gross N. J., Narine K. R. Surfactant subtypes of mice: metabolic relationships and conversion in vitro. J Appl Physiol (1985) 1989 Jul;67(1):414–421. doi: 10.1152/jappl.1989.67.1.414. [DOI] [PubMed] [Google Scholar]
- Gross N. J., Schultz R. M. Serine proteinase requirement for the extra-cellular metabolism of pulmonary surfactant. Biochim Biophys Acta. 1990 May 22;1044(2):222–230. doi: 10.1016/0005-2760(90)90306-i. [DOI] [PubMed] [Google Scholar]
- Gross N. J. Surfactant subtypes in experimental lung damage: radiation pneumonitis. Am J Physiol. 1991 Apr;260(4 Pt 1):L302–L310. doi: 10.1152/ajplung.1991.260.4.L302. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Clements J. A. Pulmonary surfactant and its apoproteins. J Clin Invest. 1990 Jul;86(1):1–6. doi: 10.1172/JCI114670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbach D. M., Waeckerle J. F. Inhalation injuries. Ann Emerg Med. 1988 Dec;17(12):1316–1320. doi: 10.1016/s0196-0644(88)80357-3. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Lewis J., Ikegami M. In vitro conversion of surfactant subtypes is altered in alveolar surfactant isolated from injured lungs. Am Rev Respir Dis. 1992 Jun;145(6):1416–1420. doi: 10.1164/ajrccm/145.6.1416. [DOI] [PubMed] [Google Scholar]
- Le Mesurier S. M., Lykke A. W., Stewart B. W. Reduced yield of pulmonary surfactant: patterns of response following administration of chemicals to rats by inhalation. Toxicol Lett. 1980 Jan;5(1):89–93. doi: 10.1016/0378-4274(80)90153-8. [DOI] [PubMed] [Google Scholar]
- Lewis J. F., Ikegami M., Jobe A. H. Altered surfactant function and metabolism in rabbits with acute lung injury. J Appl Physiol (1985) 1990 Dec;69(6):2303–2310. doi: 10.1152/jappl.1990.69.6.2303. [DOI] [PubMed] [Google Scholar]
- Magoon M. W., Wright J. R., Baritussio A., Williams M. C., Goerke J., Benson B. J., Hamilton R. L., Clements J. A. Subfractionation of lung surfactant. Implications for metabolism and surface activity. Biochim Biophys Acta. 1983 Jan 7;750(1):18–31. doi: 10.1016/0005-2760(83)90200-x. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Nellenbogen J., Clements J. A. Isolation of disaturated phosphatidylcholine with osmium tetroxide. J Lipid Res. 1976 May;17(3):281–284. [PubMed] [Google Scholar]
- Miles P. R., Bowman L., Tucker J., Reasor M. J., Wright J. R. Alterations in rat alveolar surfactant phospholipids and proteins induced by administration of chlorphentermine. Biochim Biophys Acta. 1986 Jun 11;877(1):167–178. doi: 10.1016/0005-2760(86)90132-3. [DOI] [PubMed] [Google Scholar]
- Moores H. K., Janigan D. T., Hajela R. P. Lung injury after experimental smoke inhalation: particle-associated changes in alveolar macrophages. Toxicol Pathol. 1993 Nov-Dec;21(6):521–527. doi: 10.1177/019262339302100601. [DOI] [PubMed] [Google Scholar]
- Nicholas T. E., Power J. H., Barr H. A. Effect of pattern of breathing on subfractions of surfactant in tissue and alveolar compartments of the adult rat lung. Am J Respir Cell Mol Biol. 1990 Sep;3(3):251–258. doi: 10.1165/ajrcmb/3.3.251. [DOI] [PubMed] [Google Scholar]
- Nicholas T. E., Power J. H., Barr H. A. The pulmonary consequences of a deep breath. Respir Physiol. 1982 Sep;49(3):315–324. doi: 10.1016/0034-5687(82)90119-0. [DOI] [PubMed] [Google Scholar]
- Oosting R. S., Van Iwaarden J. F., Van Bree L., Verhoef J., Van Golde L. M., Haagsman H. P. Exposure of surfactant protein A to ozone in vitro and in vivo impairs its interactions with alveolar cells. Am J Physiol. 1992 Jan;262(1 Pt 1):L63–L68. doi: 10.1152/ajplung.1992.262.1.L63. [DOI] [PubMed] [Google Scholar]
- Oulton M., Fraser M., Dolphin M., Yoon R., Faulkner G. Quantification of surfactant pool sizes in rabbit lung during perinatal development. J Lipid Res. 1986 Jun;27(6):602–612. [PubMed] [Google Scholar]
- Oulton M., MacDonald J., Janigan D. T., Faulkner G. T. Mouse alveolar surfactant: characterization of subtypes prepared by differential centrifugation. Lipids. 1993 Aug;28(8):715–720. doi: 10.1007/BF02535992. [DOI] [PubMed] [Google Scholar]
- Oulton M., Martin T. R., Faulkner G. T., Stinson D., Johnson J. P. Developmental study of a lamellar body fraction isolated from human amniotic fluid. Pediatr Res. 1980 May;14(5):722–728. doi: 10.1203/00006450-198005000-00004. [DOI] [PubMed] [Google Scholar]
- Oulton M., Moores H. K., Scott J. E., Janigan D. T., Hajela R. Effects of smoke inhalation on surfactant phospholipids and phospholipase A2 activity in the mouse lung. Am J Pathol. 1991 Jan;138(1):195–202. [PMC free article] [PubMed] [Google Scholar]
- Petraglia J. S. Fire and the aging of America. NFPA J. 1991 Mar-Apr;85(2):36-8, 41-6. [PubMed] [Google Scholar]
- Pinkerton K. E., Lewis J., Mulder A. M., Ikegami M., Jobe A. H. Surfactant treatment effects on alveolar type II cell morphology in rabbit lungs. J Appl Physiol (1985) 1993 Mar;74(3):1240–1247. doi: 10.1152/jappl.1993.74.3.1240. [DOI] [PubMed] [Google Scholar]
- Power J. H., Barr H. A., Jones M. E., Nicholas T. E. Changes in surfactant pools after a physiological increase in alveolar surfactant. J Appl Physiol (1985) 1987 Nov;63(5):1902–1911. doi: 10.1152/jappl.1987.63.5.1902. [DOI] [PubMed] [Google Scholar]
- Rider E. D., Ikegami M., Jobe A. H. Localization of alveolar surfactant clearance in rabbit lung cells. Am J Physiol. 1992 Aug;263(2 Pt 1):L201–L209. doi: 10.1152/ajplung.1992.263.2.L201. [DOI] [PubMed] [Google Scholar]
- Sanderson R. J., Vatter A. E. A mode of formation of tubular myelin from lamellar bodies in the lung. J Cell Biol. 1977 Sep;74(3):1027–1031. doi: 10.1083/jcb.74.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Forkert P. G., Oulton M., Rasmusson M. G., Temple S., Fraser M. O., Whitefield S. Pulmonary toxicity of trichloroethylene: induction of changes in surfactant phospholipids and phospholipase A2 activity in the mouse lung. Exp Mol Pathol. 1988 Aug;49(1):141–150. doi: 10.1016/0014-4800(88)90028-7. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Phosphatidylcholine synthesis, secretion, and reutilization during differentiation of the surfactant-producing type II alveolar cell from fetal rabbit lungs. Exp Lung Res. 1992 Jul-Aug;18(4):563–580. doi: 10.3109/01902149209064346. [DOI] [PubMed] [Google Scholar]
- Seeger W., Günther A., Thede C. Differential sensitivity to fibrinogen inhibition of SP-C- vs. SP-B-based surfactants. Am J Physiol. 1992 Mar;262(3 Pt 1):L286–L291. doi: 10.1152/ajplung.1992.262.3.L286. [DOI] [PubMed] [Google Scholar]
- Spain C. L., Silbajoris R., Young S. L. Alterations of surfactant pools in fetal and newborn rat lungs. Pediatr Res. 1987 Jan;21(1):5–9. doi: 10.1203/00006450-198701000-00002. [DOI] [PubMed] [Google Scholar]
- Tahvanainen J., Hallman M. Surfactant abnormality after endotoxin-induced lung injury in guinea-pigs. Eur J Respir Dis. 1987 Oct;71(4):250–258. [PubMed] [Google Scholar]
- Wright J. R., Dobbs L. G. Regulation of pulmonary surfactant secretion and clearance. Annu Rev Physiol. 1991;53:395–414. doi: 10.1146/annurev.ph.53.030191.002143. [DOI] [PubMed] [Google Scholar]



