Abstract
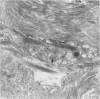
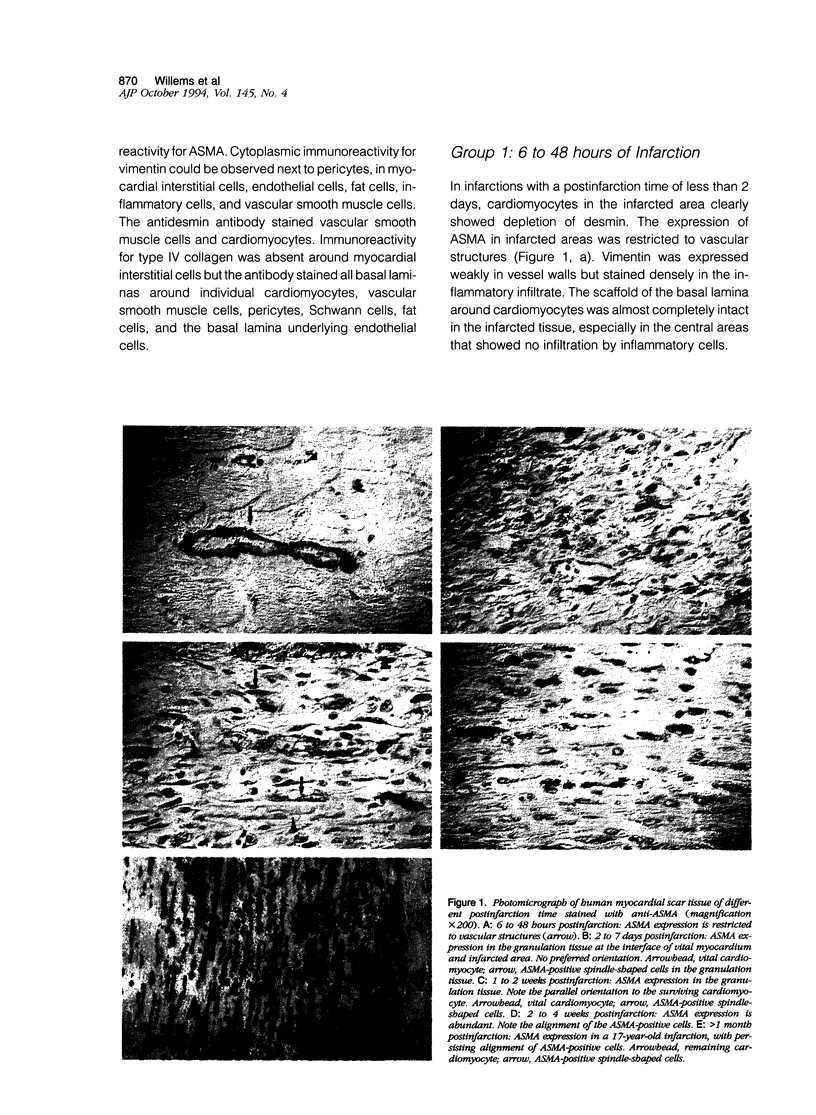
Interstitial cells in the scars of human myocardial infarctions of different postinfarction times (6 hours to 17 years old) were characterized by antibodies to alpha-smooth muscle actin (ASMA), vimentin, and desmin. Basal lamina deposition was studied with antibodies to the basal lamina protein type IV collagen. Nonvascular spindle-shaped cells expressing ASMA were present within 4 to 6 days after infarction. These cells co-expressed vimentin but no desmin and showed discontinuous basal lamina deposition. In electron microscopy these cells showed features characteristic of myofibroblasts. The spindle-shaped cells persisted for a long period of time and could even be identified 17 years postinfarction. In transmural infarctions they were orientated parallel to the endocardium and epicardium. In nontransmural patchy infarctions they showed an orientation adjacent to the cardiomyocytes and appeared to be less dense than in the transmural infarctions. In conclusion, myofibroblasts expressing ASMA persist within human myocardial scars and show a preferential alignment that may be the result of the continuous mechanical stress caused by the ongoing contraction and relaxation of the surrounding viable myocardium.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler K. B., Low R. B., Leslie K. O., Mitchell J., Evans J. N. Contractile cells in normal and fibrotic lung. Lab Invest. 1989 Apr;60(4):473–485. [PubMed] [Google Scholar]
- Amberger A., Bauer H., Tontsch U., Gabbiani G., Kocher O., Bauer H. C. Reversible expression of sm alpha-actin protein and sm alpha-actin mRNA in cloned cerebral endothelial cells. FEBS Lett. 1991 Aug 5;287(1-2):223–225. doi: 10.1016/0014-5793(91)80056-9. [DOI] [PubMed] [Google Scholar]
- Bachem M. G., Sell K. M., Melchior R., Kropf J., Eller T., Gressner A. M. Tumor necrosis factor alpha (TNF alpha) and transforming growth factor beta 1 (TGF beta 1) stimulate fibronectin synthesis and the transdifferentiation of fat-storing cells in the rat liver into myofibroblasts. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63(2):123–130. doi: 10.1007/BF02899251. [DOI] [PubMed] [Google Scholar]
- Battersby S., Anderson T. J. Myofibroblast activity of radial scars. J Pathol. 1985 Sep;147(1):33–40. doi: 10.1002/path.1711470105. [DOI] [PubMed] [Google Scholar]
- Baur P. S., Jr, Parks D. H., Hudson J. D. Epithelial mediated wound contraction in experimental wounds--the purse-string effect. J Trauma. 1984 Aug;24(8):713–720. doi: 10.1097/00005373-198408000-00004. [DOI] [PubMed] [Google Scholar]
- Bouchardy B., Majno G. Histopathology of early myocardial infarcts. A new approach. Am J Pathol. 1974 Feb;74(2):301–330. [PMC free article] [PubMed] [Google Scholar]
- Daemen M. J., Lombardi D. M., Bosman F. T., Schwartz S. M. Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res. 1991 Feb;68(2):450–456. doi: 10.1161/01.res.68.2.450. [DOI] [PubMed] [Google Scholar]
- Darby I., Skalli O., Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990 Jul;63(1):21–29. [PubMed] [Google Scholar]
- Desmoulière A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993 Jul;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmoulière A., Rubbia-Brandt L., Grau G., Gabbiani G. Heparin induces alpha-smooth muscle actin expression in cultured fibroblasts and in granulation tissue myofibroblasts. Lab Invest. 1992 Dec;67(6):716–726. [PubMed] [Google Scholar]
- Eddy R. J., Petro J. A., Tomasek J. J. Evidence for the nonmuscle nature of the "myofibroblast" of granulation tissue and hypertropic scar. An immunofluorescence study. Am J Pathol. 1988 Feb;130(2):252–260. [PMC free article] [PubMed] [Google Scholar]
- El-Labban N. G., Lee K. W. Myofibroblasts in central giant cell granuloma of the jaws: an ultrastructural study. Histopathology. 1983 Nov;7(6):907–918. doi: 10.1111/j.1365-2559.1983.tb02305.x. [DOI] [PubMed] [Google Scholar]
- Engelmann G. L., Boehm K. D., Birchenall-Roberts M. C., Ruscetti F. W. Transforming growth factor-beta 1 in heart development. Mech Dev. 1992 Aug;38(2):85–97. doi: 10.1016/0925-4773(92)90001-z. [DOI] [PubMed] [Google Scholar]
- Filip D. A., Radu A., Simionescu M. Interstitial cells of the heart valves possess characteristics similar to smooth muscle cells. Circ Res. 1986 Sep;59(3):310–320. doi: 10.1161/01.res.59.3.310. [DOI] [PubMed] [Google Scholar]
- Foo I. T., Naylor I. L., Timmons M. J., Trejdosiewicz L. K. Intracellular actin as a marker for myofibroblasts in vitro. Lab Invest. 1992 Dec;67(6):727–733. [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971 May 15;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Havenith M. G., Cleutjens J. P., Beek C., vd Linden E., De Goeij A. F., Bosman F. T. Human specific anti-type IV collagen monoclonal antibodies, characterization and immunohistochemical application. Histochemistry. 1987;87(2):123–128. doi: 10.1007/BF00533396. [DOI] [PubMed] [Google Scholar]
- Heine U. I., Burmester J. K., Flanders K. C., Danielpour D., Munoz E. F., Roberts A. B., Sporn M. B. Localization of transforming growth factor-beta 1 in mitochondria of murine heart and liver. Cell Regul. 1991 Jun;2(6):467–477. doi: 10.1091/mbc.2.6.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapanci Y., Assimacopoulos A., Irle C., Zwahlen A., Gabbiani G. "Contractile interstitial cells" in pulmonary alveolar septa: a possible regulator of ventilation-perfusion ratio? Ultrastructural, immunofluorescence, and in vitro studies. J Cell Biol. 1974 Feb;60(2):375–392. doi: 10.1083/jcb.60.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie K. O., Taatjes D. J., Schwarz J., vonTurkovich M., Low R. B. Cardiac myofibroblasts express alpha smooth muscle actin during right ventricular pressure overload in the rabbit. Am J Pathol. 1991 Jul;139(1):207–216. [PMC free article] [PubMed] [Google Scholar]
- Lipper S., Kahn L. B., Reddick R. L. The myofibroblast. Pathol Annu. 1980;15(Pt 1):409–441. [PubMed] [Google Scholar]
- MacLellan W. R., Brand T., Schneider M. D. Transforming growth factor-beta in cardiac ontogeny and adaptation. Circ Res. 1993 Nov;73(5):783–791. doi: 10.1161/01.res.73.5.783. [DOI] [PubMed] [Google Scholar]
- Matsuoka L. Y., Uitto J., Wortsman J., Abergel R. P., Dietrich J. Ultrastructural characteristics of keloid fibroblasts. Am J Dermatopathol. 1988 Dec;10(6):505–508. doi: 10.1097/00000372-198812000-00005. [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Vande Berg J., Rudolph R., Tarpley J., Mustoe T. A. Platelet-derived growth factor-BB and transforming growth factor beta 1 selectively modulate glycosaminoglycans, collagen, and myofibroblasts in excisional wounds. Am J Pathol. 1991 Mar;138(3):629–646. [PMC free article] [PubMed] [Google Scholar]
- Pourreau-Schneider N., Ahmed A., Soudry M., Jacquemier J., Kopp F., Franquin J. C., Martin P. M. Helium-neon laser treatment transforms fibroblasts into myofibroblasts. Am J Pathol. 1990 Jul;137(1):171–178. [PMC free article] [PubMed] [Google Scholar]
- Schürch W., Seemayer T. A., Lagacé R., Gabbiani G. The intermediate filament cytoskeleton of myofibroblasts: an immunofluorescence and ultrastructural study. Virchows Arch A Pathol Anat Histopathol. 1984;403(4):323–336. doi: 10.1007/BF00737283. [DOI] [PubMed] [Google Scholar]
- Seemayer T. A., Lagacé R., Schürch W., Thelmo W. L. The myofibroblast: biologic, pathologic, and theoretical considerations. Pathol Annu. 1980;15(Pt 1):443–470. [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Schürch W., Seemayer T., Lagacé R., Montandon D., Pittet B., Gabbiani G. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest. 1989 Feb;60(2):275–285. [PubMed] [Google Scholar]
- Sottiurai V. S., Batson R. C. Role of myofibroblasts in pseudointima formation. Surgery. 1983 Nov;94(5):792–801. [PubMed] [Google Scholar]
- Stout L. C., Boor P. J., Whorton E. B., Jr Myofibroblastic proliferation on mitral valve chordae tendineae: a distinctive lesion associated with alcoholic liver disease. Hum Pathol. 1988 Jun;19(6):720–725. doi: 10.1016/s0046-8177(88)80179-5. [DOI] [PubMed] [Google Scholar]
- Thiemermann C., Corder R. Is endothelin-1 the regulator of myofibroblast contraction during wound healing? Lab Invest. 1992 Dec;67(6):677–679. [PubMed] [Google Scholar]
- Thompson N. L., Bazoberry F., Speir E. H., Casscells W., Ferrans V. J., Flanders K. C., Kondaiah P., Geiser A. G., Sporn M. B. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988;1(1):91–99. doi: 10.3109/08977198809000251. [DOI] [PubMed] [Google Scholar]
- Tomasek J. J., Schultz R. J., Episalla C. W., Newman S. A. The cytoskeleton and extracellular matrix of the Dupuytren's disease "myofibroblast": an immunofluorescence study of a nonmuscle cell type. J Hand Surg Am. 1986 May;11(3):365–371. doi: 10.1016/s0363-5023(86)80143-5. [DOI] [PubMed] [Google Scholar]
- Van Muijen G. N., Ruiter D. J., Warnaar S. O. Coexpression of intermediate filament polypeptides in human fetal and adult tissues. Lab Invest. 1987 Oct;57(4):359–369. [PubMed] [Google Scholar]
- Vande Berg J. S., Rudolph R., Woodward M. Growth dynamics of cultured myofibroblasts from human breast cancer and nonmalignant contracting tissues. Plast Reconstr Surg. 1984 Apr;73(4):605–618. doi: 10.1097/00006534-198404000-00016. [DOI] [PubMed] [Google Scholar]
- Volders P. G., Willems I. E., Cleutjens J. P., Arends J. W., Havenith M. G., Daemen M. J. Interstitial collagen is increased in the non-infarcted human myocardium after myocardial infarction. J Mol Cell Cardiol. 1993 Nov;25(11):1317–1323. doi: 10.1006/jmcc.1993.1144. [DOI] [PubMed] [Google Scholar]
- Vracko R., Cunningham D., Frederickson R. G., Thorning D. Basal lamina of rat myocardium. Its fate after death of cardiac myocytes. Lab Invest. 1988 Jan;58(1):77–87. [PubMed] [Google Scholar]
- Vracko R., Thorning D. Contractile cells in rat myocardial scar tissue. Lab Invest. 1991 Aug;65(2):214–227. [PubMed] [Google Scholar]
- Vracko R., Thorning D., Frederickson R. G. Connective tissue cells in healing rat myocardium. A study of cell reactions in rhythmically contracting environment. Am J Pathol. 1989 May;134(5):993–1006. [PMC free article] [PubMed] [Google Scholar]
- Vyalov S., Desmoulière A., Gabbiani G. GM-CSF-induced granulation tissue formation: relationships between macrophage and myofibroblast accumulation. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63(4):231–239. doi: 10.1007/BF02899267. [DOI] [PubMed] [Google Scholar]
- van Krimpen C., Schoemaker R. G., Cleutjens J. P., Smits J. F., Struyker-Boudier H. A., Bosman F. T., Daemen M. J. Angiotensin I converting enzyme inhibitors and cardiac remodeling. Basic Res Cardiol. 1991;86 (Suppl 1):149–155. [PubMed] [Google Scholar]