Abstract
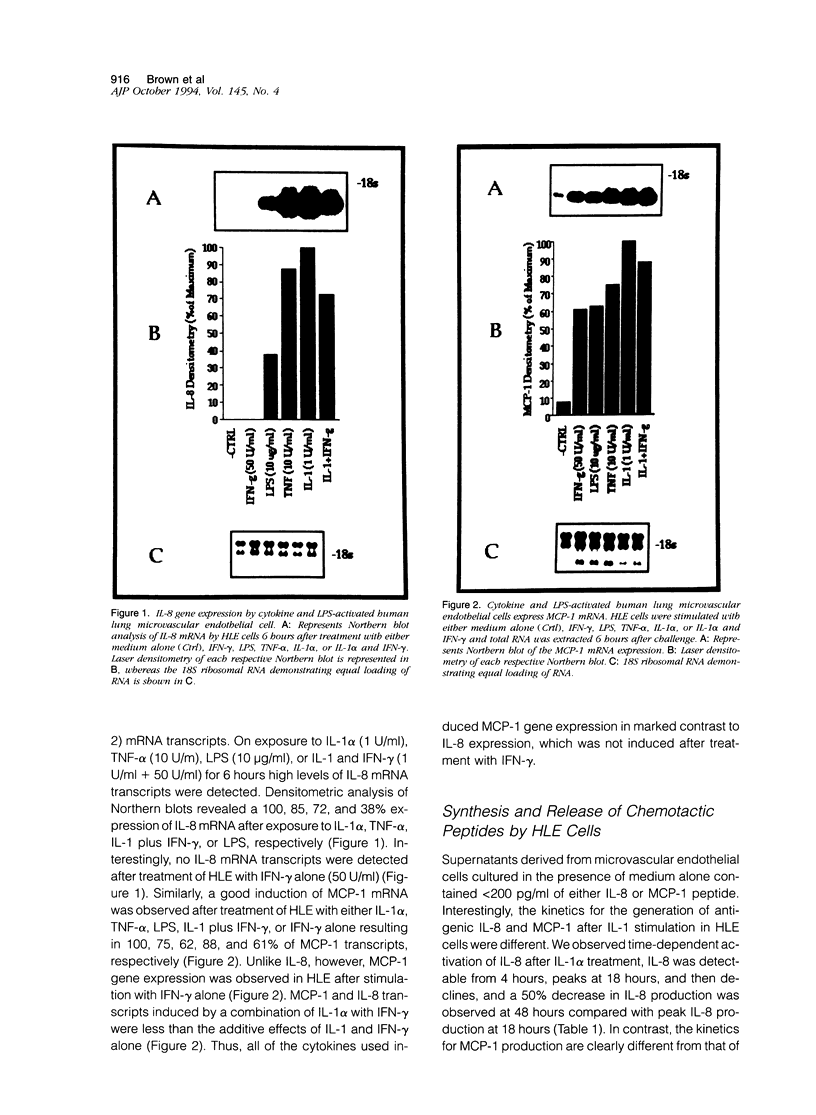
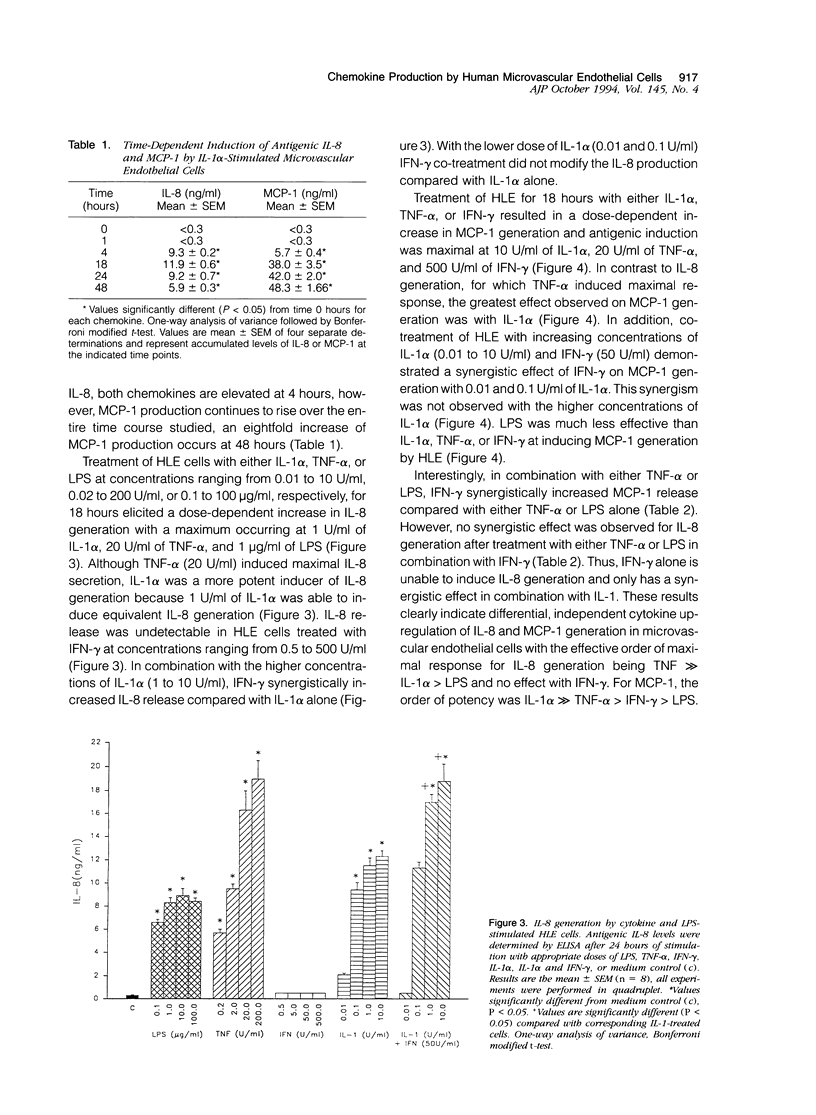
The elicitation of leukocytes from the circulation to inflamed tissue depends on the activation of both the leukocyte and endothelial cell. In this study we determined the gene expression and secretion patterns for the chemokines interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1) in cytokine- and lipopolysaccharide (LPS)-treated cultured human lung microvascular endothelial cells (HLE). HLE constitutively expressed low levels of MCP-1 and IL-8. Treatment of HLE with a variety of cytokines and LPS up-regulated both IL-8 mRNA expression and release of immunoreactive IL-8 with an order of potency tumor necrosis factor-alpha (TNF-alpha) >> IL-1 alpha > LPS, whereas interferon-gamma (IFN-gamma) had no effect on IL-8 mRNA or antigenic levels. However, IFN-gamma, in combination with high doses of IL-1 alpha, resulted in a synergistic increase in IL-8 generation. MCP-1 gene expression and secretion was induced in a dose-dependent manner after IL-1 alpha, TNF-alpha, IFN-gamma, and LPS activation of HLE. IL-1 alpha was the most potent inducer of MCP-1 generation and LPS was relatively ineffective. IFN-gamma, in combination with low doses of IL-1 alpha, resulted in a synergistic increase in MCP-1 generation by HLE. These results demonstrate that although IL-8 and MCP-1 generation by HLE occurs on cytokine treatment, the relative ability of a given cytokine to elicit IL-8 generation is not directly parallel to effects on MCP-1 generation. These data suggest that the regulation of IL-8 and MCP-1 expression exhibit significant differences in their mechanisms. Such differences in the expression of specific chemokines may explain the specific appearance of various leukocytes at sites of inflammation and injury. These data also directly demonstrate that the lung microvascular endothelium contribute to the cytokine network of the lung, with the ability to respond to locally generated cytokines and to produce potent mediators of the local inflammatory response.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Neville-Golden J., Galanopoulos T., Kradin R. L., Valente A. J., Graves D. T. Expression of monocyte chemoattractant protein 1 mRNA in human idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5371–5375. doi: 10.1073/pnas.89.12.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon K. B., Westwick J., Camp R. D. Potent and specific inhibition of IL-8-, IL-1 alpha- and IL-1 beta-induced in vitro human lymphocyte migration by calcium channel antagonists. Biochem Biophys Res Commun. 1989 Nov 30;165(1):349–354. doi: 10.1016/0006-291x(89)91076-0. [DOI] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Carley W. W., Niedbala M. J., Gerritsen M. E. Isolation, cultivation, and partial characterization of microvascular endothelium derived from human lung. Am J Respir Cell Mol Biol. 1992 Dec;7(6):620–630. doi: 10.1165/ajrcmb/7.6.620. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- D'Amore P. A., Glaser B. M., Brunson S. K., Fenselau A. H. Angiogenic activity from bovine retina: partial purification and characterization. Proc Natl Acad Sci U S A. 1981 May;78(5):3068–3072. doi: 10.1073/pnas.78.5.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanoff H. L., Burdick M. D., Moore S. A., Kunkel S. L., Strieter R. M. A sensitive ELISA for the detection of human monocyte chemoattractant protein-1 (MCP-1). Immunol Invest. 1992 Feb;21(1):39–45. doi: 10.3109/08820139209069361. [DOI] [PubMed] [Google Scholar]
- Furutani Y., Nomura H., Notake M., Oyamada Y., Fukui T., Yamada M., Larsen C. G., Oppenheim J. J., Matsushima K. Cloning and sequencing of the cDNA for human monocyte chemotactic and activating factor (MCAF). Biochem Biophys Res Commun. 1989 Feb 28;159(1):249–255. doi: 10.1016/0006-291x(89)92430-3. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Obin M. S., Brock A. F., Luis E. A., Hass P. E., Hébert C. A., Yip Y. K., Leung D. W., Lowe D. G., Kohr W. J. Endothelial interleukin-8: a novel inhibitor of leukocyte-endothelial interactions. Science. 1989 Dec 22;246(4937):1601–1603. doi: 10.1126/science.2688092. [DOI] [PubMed] [Google Scholar]
- Hechtman D. H., Cybulsky M. I., Fuchs H. J., Baker J. B., Gimbrone M. A., Jr Intravascular IL-8. Inhibitor of polymorphonuclear leukocyte accumulation at sites of acute inflammation. J Immunol. 1991 Aug 1;147(3):883–892. [PubMed] [Google Scholar]
- Huber A. R., Kunkel S. L., Todd R. F., 3rd, Weiss S. J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991 Oct 4;254(5028):99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- Hébert C. A., Luscinskas F. W., Kiely J. M., Luis E. A., Darbonne W. C., Bennett G. L., Liu C. C., Obin M. S., Gimbrone M. A., Jr, Baker J. B. Endothelial and leukocyte forms of IL-8. Conversion by thrombin and interactions with neutrophils. J Immunol. 1990 Nov 1;145(9):3033–3040. [PubMed] [Google Scholar]
- Johnson D. R., Pober J. S. Tumor necrosis factor and immune interferon synergistically increase transcription of HLA class I heavy- and light-chain genes in vascular endothelium. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5183–5187. doi: 10.1073/pnas.87.13.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas E., Sargent T. D., Dawid I. B. Epidermal keratin gene expressed in embryos of Xenopus laevis. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5413–5417. doi: 10.1073/pnas.82.16.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Leonard E. J., Skeel A., Yoshimura T., Noer K., Kutvirt S., Van Epps D. Leukocyte specificity and binding of human neutrophil attractant/activation protein-1. J Immunol. 1990 Feb 15;144(4):1323–1330. [PubMed] [Google Scholar]
- Mantovani A., Bussolino F., Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992 May;6(8):2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Oppenheim J. J. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989 Nov;1(1):2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- Mukaida N., Shiroo M., Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989 Aug 15;143(4):1366–1371. [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Preiser J. C., Schmartz D., Van der Linden P., Content J., Vanden Bussche P., Buurman W., Sebald W., Dupont E., Pinsky M. R., Vincent J. L. Interleukin-6 administration has no acute hemodynamic or hematologic effect in the dog. Cytokine. 1991 Jan;3(1):1–4. doi: 10.1016/1043-4666(91)90002-u. [DOI] [PubMed] [Google Scholar]
- Robinson B. W., McLemore T. L., Crystal R. G. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985 May;75(5):1488–1495. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe M. W., Kunkel S. L., Standiford T. J., Chensue S. W., Allen R. M., Evanoff H. L., Phan S. H., Strieter R. M. Pulmonary fibroblast expression of interleukin-8: a model for alveolar macrophage-derived cytokine networking. Am J Respir Cell Mol Biol. 1991 Nov;5(5):493–501. doi: 10.1165/ajrcmb/5.5.493. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Pober J. S. Interleukin-4 induces the synthesis and secretion of MCP-1/JE by human endothelial cells. Am J Pathol. 1991 Jun;138(6):1315–1319. [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Stier P., Ernst T., Wong G. G. The human homolog of the JE gene encodes a monocyte secretory protein. Mol Cell Biol. 1989 Nov;9(11):4687–4695. doi: 10.1128/mcb.9.11.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Yoshimura T., Leonard E. J., Pober J. S. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990 Jun;136(6):1229–1233. [PMC free article] [PubMed] [Google Scholar]
- Schmouder R. L., Strieter R. M., Kunkel S. L. Interferon-gamma regulation of human renal cortical epithelial cell-derived monocyte chemotactic peptide-1. Kidney Int. 1993 Jul;44(1):43–49. doi: 10.1038/ki.1993.211. [DOI] [PubMed] [Google Scholar]
- Schröder J. M., Christophers E. Secretion of novel and homologous neutrophil-activating peptides by LPS-stimulated human endothelial cells. J Immunol. 1989 Jan 1;142(1):244–251. [PubMed] [Google Scholar]
- Shyy Y. J., Li Y. S., Kolattukudy P. E. Structure of human monocyte chemotactic protein gene and its regulation by TPA. Biochem Biophys Res Commun. 1990 Jun 15;169(2):346–351. doi: 10.1016/0006-291x(90)90338-n. [DOI] [PubMed] [Google Scholar]
- Sica A., Matsushima K., Van Damme J., Wang J. M., Polentarutti N., Dejana E., Colotta F., Mantovani A. IL-1 transcriptionally activates the neutrophil chemotactic factor/IL-8 gene in endothelial cells. Immunology. 1990 Apr;69(4):548–553. [PMC free article] [PubMed] [Google Scholar]
- Sica A., Wang J. M., Colotta F., Dejana E., Mantovani A., Oppenheim J. J., Larsen C. G., Zachariae C. O., Matsushima K. Monocyte chemotactic and activating factor gene expression induced in endothelial cells by IL-1 and tumor necrosis factor. J Immunol. 1990 Apr 15;144(8):3034–3038. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Basha M. A., Chensue S. W., Lynch J. P., 3rd, Toews G. B., Westwick J., Strieter R. M. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990 Dec;86(6):1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter R. M., Chensue S. W., Basha M. A., Standiford T. J., Lynch J. P., Baggiolini M., Kunkel S. L. Human alveolar macrophage gene expression of interleukin-8 by tumor necrosis factor-alpha, lipopolysaccharide, and interleukin-1 beta. Am J Respir Cell Mol Biol. 1990 Apr;2(4):321–326. doi: 10.1165/ajrcmb/2.4.321. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Showell H. J., Remick D. G., Phan S. H., Ward P. A., Marks R. M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989 Mar 17;243(4897):1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Phan S. H., Showell H. J., Remick D. G., Lynch J. P., Genord M., Raiford C., Eskandari M., Marks R. M., Kunkel S. L. Monokine-induced neutrophil chemotactic factor gene expression in human fibroblasts. J Biol Chem. 1989 Jun 25;264(18):10621–10626. [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright T. M., Farber J. M. 5' regulatory region of a novel cytokine gene mediates selective activation by interferon gamma. J Exp Med. 1991 Feb 1;173(2):417–422. doi: 10.1084/jem.173.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Yuhki N., Moore S. K., Appella E., Lerman M. I., Leonard E. J. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989 Feb 27;244(2):487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]



