Abstract
In the present study, we examined the distribution of integrin subunits and extracellular matrix proteins in normal testis, intratubular germ cell neoplasia (ITGCN), and primary and metastatic seminomas. Compared to normal testis in ITGCN, Sertoli cells showed increased expression of alpha 3, alpha 6, and beta 1 integrin subunits. Malignant intratubular germ cells stained for alpha 3, alpha 6, and beta 1 integrin subunits. Progression of ITGCN to invasive seminoma was associated with loss of alpha 3 integrin subunit expression by tumor cells. Consequent to this loss, it can be speculated that the strong expression on ITGCN may be related to the noninvasive character of the lesion as is also known from other noninvasive tumors. All tumors showed a strong expression of alpha 6 and beta 1 integrin subunits. The alpha 5 integrin subunit was weakly expressed in primary seminomas in all stages. No differences were observed in integrin expression between primary and metastatic tumors. The distribution of extracellular matrix proteins was heterogeneous and revealed clear architectural differences between seminomas that may reflect different stages of tumor stroma formation. To our knowledge, the results presented in this study provide the first information on the possible role of tumor-extracellular matrix interactions in the biological behavior of ITGCN and testicular seminomas.
Full text
PDF



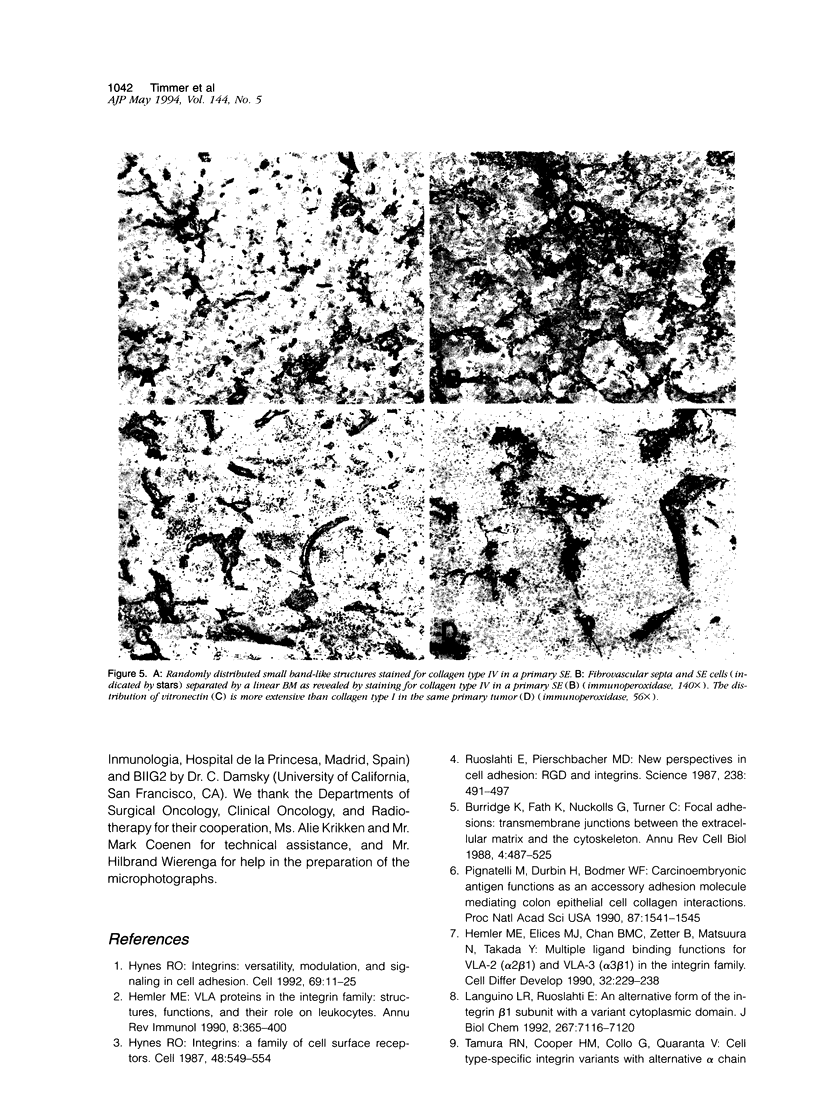


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M. Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest. 1993 Jan;68(1):4–17. [PubMed] [Google Scholar]
- Argraves W. S., Suzuki S., Arai H., Thompson K., Pierschbacher M. D., Ruoslahti E. Amino acid sequence of the human fibronectin receptor. J Cell Biol. 1987 Sep;105(3):1183–1190. doi: 10.1083/jcb.105.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsky S. H., Siegal G. P., Jannotta F., Liotta L. A. Loss of basement membrane components by invasive tumors but not by their benign counterparts. Lab Invest. 1983 Aug;49(2):140–147. [PubMed] [Google Scholar]
- Bauer J. S., Schreiner C. L., Giancotti F. G., Ruoslahti E., Juliano R. L. Motility of fibronectin receptor-deficient cells on fibronectin and vitronectin: collaborative interactions among integrins. J Cell Biol. 1992 Jan;116(2):477–487. doi: 10.1083/jcb.116.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Bosman F. T., Havenith M. G., Visser R., Cleutjens J. P. Basement membranes in neoplasia. Prog Histochem Cytochem. 1992;24(4):1–92. doi: 10.1016/s0079-6336(11)80212-3. [DOI] [PubMed] [Google Scholar]
- Brown D. L., Phillips D. R., Damsky C. H., Charo I. F. Synthesis and expression of the fibroblast fibronectin receptor in human monocytes. J Clin Invest. 1989 Jul;84(1):366–370. doi: 10.1172/JCI114166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. F., Lanir N., McDonagh J., Tognazzi K., Dvorak A. M., Dvorak H. F. Fibroblast migration in fibrin gel matrices. Am J Pathol. 1993 Jan;142(1):273–283. [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Cassiman J. J. The involvement of the cell matrix receptors, or VLA integrins, in the morphogenetic behavior of normal and malignant cells is gradually being uncovered. Cancer Genet Cytogenet. 1989 Aug;41(1):19–32. doi: 10.1016/0165-4608(89)90104-0. [DOI] [PubMed] [Google Scholar]
- Chan B. M., Matsuura N., Takada Y., Zetter B. R., Hemler M. E. In vitro and in vivo consequences of VLA-2 expression on rhabdomyosarcoma cells. Science. 1991 Mar 29;251(5001):1600–1602. doi: 10.1126/science.2011740. [DOI] [PubMed] [Google Scholar]
- Cooper S., Pera M. F. Vitronectin production by human yolk sac carcinoma cells resembling parietal endoderm. Development. 1988 Dec;104(4):565–574. doi: 10.1242/dev.104.4.565. [DOI] [PubMed] [Google Scholar]
- Cornil I., Theodorescu D., Man S., Herlyn M., Jambrosic J., Kerbel R. S. Fibroblast cell interactions with human melanoma cells affect tumor cell growth as a function of tumor progression. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6028–6032. doi: 10.1073/pnas.88.14.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross M., Dexter T. M. Growth factors in development, transformation, and tumorigenesis. Cell. 1991 Jan 25;64(2):271–280. doi: 10.1016/0092-8674(91)90638-f. [DOI] [PubMed] [Google Scholar]
- Dedhar S., Saulnier R. Alterations in integrin receptor expression on chemically transformed human cells: specific enhancement of laminin and collagen receptor complexes. J Cell Biol. 1990 Feb;110(2):481–489. doi: 10.1083/jcb.110.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Form D. M., Manseau E. J., Smith B. D. Pathogenesis of desmoplasia. I. Immunofluorescence identification and localization of some structural proteins of line 1 and line 10 guinea pig tumors and of healing wounds. J Natl Cancer Inst. 1984 Nov;73(5):1195–1205. [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Enders G. C., Henson J. H., Millette C. F. Sertoli cell binding to isolated testicular basement membrane. J Cell Biol. 1986 Sep;103(3):1109–1119. doi: 10.1083/jcb.103.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler I. J. In vitro studies of cellular-mediated immunostimulation of tumor growth. J Natl Cancer Inst. 1973 May;50(5):1307–1312. doi: 10.1093/jnci/50.5.1307. [DOI] [PubMed] [Google Scholar]
- Fradet Y., Cordon-Cardo C., Thomson T., Daly M. E., Whitmore W. F., Jr, Lloyd K. O., Melamed M. R., Old L. J. Cell surface antigens of human bladder cancer defined by mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1984 Jan;81(1):224–228. doi: 10.1073/pnas.81.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990 Mar 9;60(5):849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Giltay J. C., Brinkman H. J., Modderman P. W., von dem Borne A. E., van Mourik J. A. Human vascular endothelial cells express a membrane protein complex immunochemically indistinguishable from the platelet VLA-2 (glycoprotein Ia-IIa) complex. Blood. 1989 Apr;73(5):1235–1241. [PubMed] [Google Scholar]
- Havenith M. G., Dingemans K. P., Cleutjens J. P., Wagenaar S. S., Bosman F. T. Basement membranes in bronchogenic squamous cell carcinoma: an immunohistochemical and ultrastructural study. Ultrastruct Pathol. 1990 Jan-Feb;14(1):51–63. doi: 10.3109/01913129009050874. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Elices M. J., Chan B. M., Zetter B., Matsuura N., Takada Y. Multiple ligand binding functions for VLA-2 (alpha 2 beta 1) and VLA-3 (alpha 3 beta 1) in the integrin family. Cell Differ Dev. 1990 Dec 2;32(3):229–238. doi: 10.1016/0922-3371(90)90035-u. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Takada Y., Schwarz L., Strominger J. L., Clabby M. L. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem. 1987 Aug 25;262(24):11478–11485. [PubMed] [Google Scholar]
- Hemler M. E., Sanchez-Madrid F., Flotte T. J., Krensky A. M., Burakoff S. J., Bhan A. K., Springer T. A., Strominger J. L. Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol. 1984 Jun;132(6):3011–3018. [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Ware C. F., Strominger J. L. Characterization of a novel differentiation antigen complex recognize by a monoclonal antibody (A-1A5): unique activation-specific molecular forms on stimulated T cells. J Immunol. 1983 Jul;131(1):334–340. [PubMed] [Google Scholar]
- Herlyn M., Malkowicz S. B. Regulatory pathways in tumor growth and invasion. Lab Invest. 1991 Sep;65(3):262–271. [PubMed] [Google Scholar]
- Hessle H., Sakai L. Y., Hollister D. W., Burgeson R. E., Engvall E. Basement membrane diversity detected by monoclonal antibodies. Differentiation. 1984;26(1):49–54. doi: 10.1111/j.1432-0436.1984.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Hirst R., Horwitz A., Buck C., Rohrschneider L. Phosphorylation of the fibronectin receptor complex in cells transformed by oncogenes that encode tyrosine kinases. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6470–6474. doi: 10.1073/pnas.83.17.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honn K. V., Tang D. G. Adhesion molecules and tumor cell interaction with endothelium and subendothelial matrix. Cancer Metastasis Rev. 1992 Nov;11(3-4):353–375. doi: 10.1007/BF01307187. [DOI] [PubMed] [Google Scholar]
- Humphries M. J. Peptide recognition motifs involved in the binding of integrins to their ligands. Kidney Int. 1992 Mar;41(3):645–649. doi: 10.1038/ki.1992.99. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V. Proteoglycans: structure, function, and role in neoplasia. Lab Invest. 1985 Oct;53(4):373–396. [PubMed] [Google Scholar]
- Jacobsen G. K., Henriksen O. B., von der Maase H. Carcinoma in situ of testicular tissue adjacent to malignant germ-cell tumors: a study of 105 cases. Cancer. 1981 Jun 1;47(11):2660–2662. doi: 10.1002/1097-0142(19810601)47:11<2660::aid-cncr2820471123>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L. Membrane receptors for extracellular matrix macromolecules: relationship to cell adhesion and tumor metastasis. Biochim Biophys Acta. 1987 Nov 25;907(3):261–278. doi: 10.1016/0304-419x(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Jørgensen N., Müller J., Giwercman A., Skakkebaek N. E. Clinical and biological significance of carcinoma in situ of the testis. Cancer Surv. 1990;9(2):287–302. [PubMed] [Google Scholar]
- Kimmel K. A., Carey T. E. Altered expression in squamous carcinoma cells of an orientation restricted epithelial antigen detected by monoclonal antibody A9. Cancer Res. 1986 Jul;46(7):3614–3623. [PubMed] [Google Scholar]
- Languino L. R., Ruoslahti E. An alternative form of the integrin beta 1 subunit with a variant cytoplasmic domain. J Biol Chem. 1992 Apr 5;267(10):7116–7120. [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- McCarthy J. B., Skubitz A. P., Palm S. L., Furcht L. T. Metastasis inhibition of different tumor types by purified laminin fragments and a heparin-binding fragment of fibronectin. J Natl Cancer Inst. 1988 Mar 16;80(2):108–116. doi: 10.1093/jnci/80.2.108. [DOI] [PubMed] [Google Scholar]
- Mikulowski P., Oldbring J. Microinvasive germ cell neoplasia of the testis. Cancer. 1992 Aug 1;70(3):659–664. doi: 10.1002/1097-0142(19920801)70:3<659::aid-cncr2820700320>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Nagy J. A., Brown L. F., Senger D. R., Lanir N., Van de Water L., Dvorak A. M., Dvorak H. F. Pathogenesis of tumor stroma generation: a critical role for leaky blood vessels and fibrin deposition. Biochim Biophys Acta. 1989 Feb;948(3):305–326. doi: 10.1016/0304-419x(89)90004-8. [DOI] [PubMed] [Google Scholar]
- Pauli B. U., Knudson W. Tumor invasion: a consequence of destructive and compositional matrix alterations. Hum Pathol. 1988 Jun;19(6):628–639. doi: 10.1016/s0046-8177(88)80168-0. [DOI] [PubMed] [Google Scholar]
- Pierce G. B., Jr Ultrastructure of human testicular tumors. Cancer. 1966 Dec;19(12):1963–1983. doi: 10.1002/1097-0142(196612)19:12<1963::aid-cncr2820191224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Pignatelli M., Cardillo M. R., Hanby A., Stamp G. W. Integrins and their accessory adhesion molecules in mammary carcinomas: loss of polarization in poorly differentiated tumors. Hum Pathol. 1992 Oct;23(10):1159–1166. doi: 10.1016/0046-8177(92)90034-z. [DOI] [PubMed] [Google Scholar]
- Pignatelli M., Durbin H., Bodmer W. F. Carcinoembryonic antigen functions as an accessory adhesion molecule mediating colon epithelial cell-collagen interactions. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1541–1545. doi: 10.1073/pnas.87.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantefaber L. C., Hynes R. O. Changes in integrin receptors on oncogenically transformed cells. Cell. 1989 Jan 27;56(2):281–290. doi: 10.1016/0092-8674(89)90902-1. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Pöllänen P. P., Kallajoki M., Risteli L., Risteli J., Suominen J. J. Laminin and type IV collagen in the human testis. Int J Androl. 1985 Oct;8(5):337–347. doi: 10.1111/j.1365-2605.1985.tb00846.x. [DOI] [PubMed] [Google Scholar]
- Rabes H. M., Schmeller N., Hartmann A., Rattenhuber U., Carl P., Staehler G. Analysis of proliferative compartments in human tumors. II. Seminoma. Cancer. 1985 Apr 15;55(8):1758–1769. doi: 10.1002/1097-0142(19850415)55:8<1758::aid-cncr2820550824>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Santamaria L., Martinez-Onsurbe P., Paniagua R., Nistal M. Laminin, type IV collagen, and fibronectin in normal and cryptorchid human testes. An immunohistochemical study. Int J Androl. 1990 Apr;13(2):135–146. doi: 10.1111/j.1365-2605.1990.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Schreiner C., Fisher M., Hussein S., Juliano R. L. Increased tumorigenicity of fibronectin receptor deficient Chinese hamster ovary cell variants. Cancer Res. 1991 Mar 15;51(6):1738–1740. [PubMed] [Google Scholar]
- Schulze C., Holstein A. F. On the histology of human seminoma: development of the solid tumor from intratubular seminoma cells. Cancer. 1977 Mar;39(3):1090–1100. doi: 10.1002/1097-0142(197703)39:3<1090::aid-cncr2820390313>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A. Signaling by integrins: implications for tumorigenesis. Cancer Res. 1993 Apr 1;53(7):1503–1506. [PubMed] [Google Scholar]
- Skakkebaek N. E., Berthelsen J. G., Giwercman A., Müller J. Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl. 1987 Feb;10(1):19–28. doi: 10.1111/j.1365-2605.1987.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Janssen H., Hogervorst F., Calafat J., Hilgers J. A complex of platelet glycoproteins Ic and IIa identified by a rat monoclonal antibody. J Biol Chem. 1987 Jul 25;262(21):10376–10383. [PubMed] [Google Scholar]
- Sonnenberg A., Modderman P. W., Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988 Dec 1;336(6198):487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Stamp G. W., Pignatelli M. Distribution of beta 1, alpha 1, alpha 2 and alpha 3 integrin chains in basal cell carcinomas. J Pathol. 1991 Apr;163(4):307–313. doi: 10.1002/path.1711630407. [DOI] [PubMed] [Google Scholar]
- Streuli C. H., Schmidhauser C., Kobrin M., Bissell M. J., Derynck R. Extracellular matrix regulates expression of the TGF-beta 1 gene. J Cell Biol. 1993 Jan;120(1):253–260. doi: 10.1083/jcb.120.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Madrid F., De Landázuri M. O., Morago G., Cebrián M., Acevedo A., Bernabeu C. VLA-3: a novel polypeptide association within the VLA molecular complex: cell distribution and biochemical characterization. Eur J Immunol. 1986 Nov;16(11):1343–1349. doi: 10.1002/eji.1830161106. [DOI] [PubMed] [Google Scholar]
- Tomasini B. R., Mosher D. F. Vitronectin. Prog Hemost Thromb. 1991;10:269–305. [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G., Piotrowicz R. S., Kunicki T. J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988 Nov;107(5):1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Kleinman H. K. Functional domains of cell adhesion molecules. Curr Opin Cell Biol. 1992 Oct;4(5):819–823. doi: 10.1016/0955-0674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- van den Hooff A. Stromal involvement in malignant growth. Adv Cancer Res. 1988;50:159–196. doi: 10.1016/s0065-230x(08)60437-6. [DOI] [PubMed] [Google Scholar]