Abstract
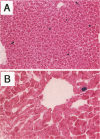
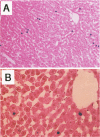
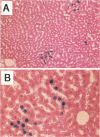
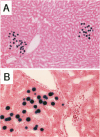
The fate of normal hepatocytes in adult rat liver was studied after genetic labeling using the Escherichia coli beta-galactosidase gene coupled to a nuclear localization signal. The marker gene was introduced by direct in vivo retroviral-mediated gene transfer into hepatocytes 24 hours after partial hepatectomy. Analysis of beta-galactosidase expression in the liver at various time after gene transfer revealed that labeled hepatocytes were distributed throughout the entire lobule with a predominance in the periportal and mediolobular regions. Long-term experiments demonstrated that division of hepatocytes did occur as was revealed by the increasing number of beta-galactosidase-positive cells in isolated clusters. There was no evidence for the participation of stem cells in this process. Moreover, we found that after more than 1 year, the pattern of distribution of positive cells within the lobule was not modified. This suggests that hepatocytes do not migrate from the portal space to the perivenous region, as has been previously hypothesized.
Full text
PDF

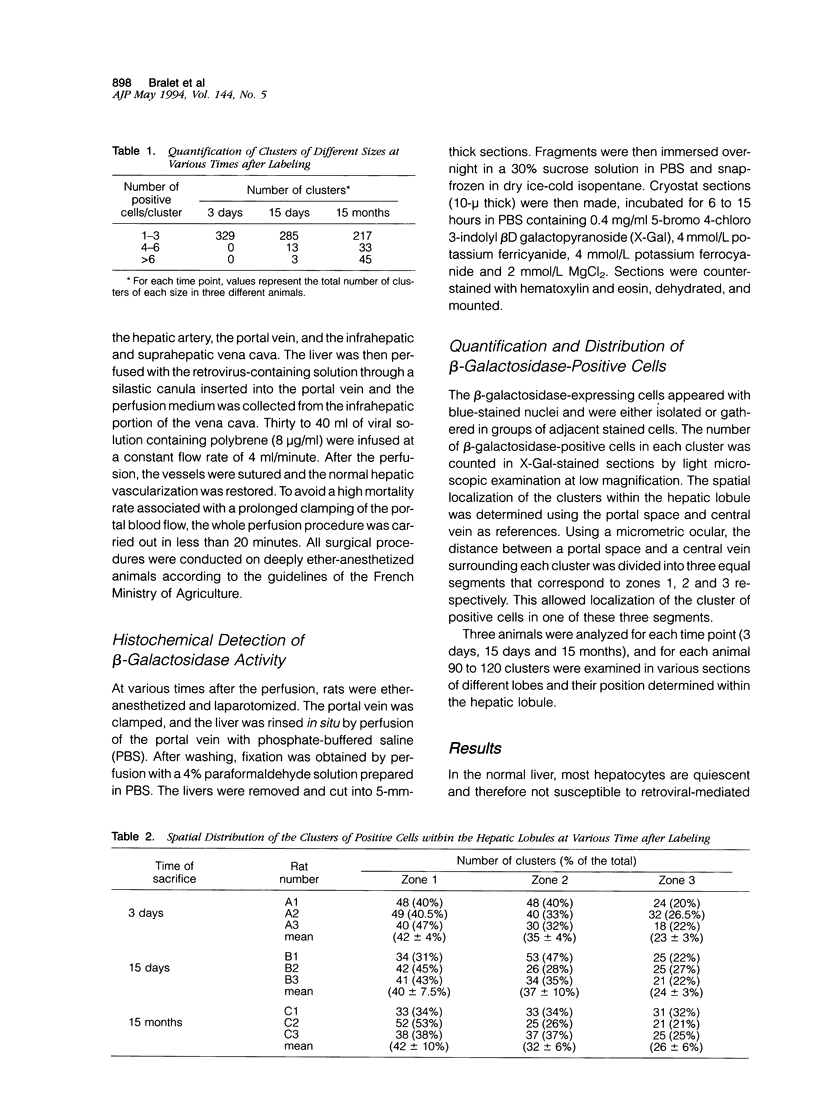
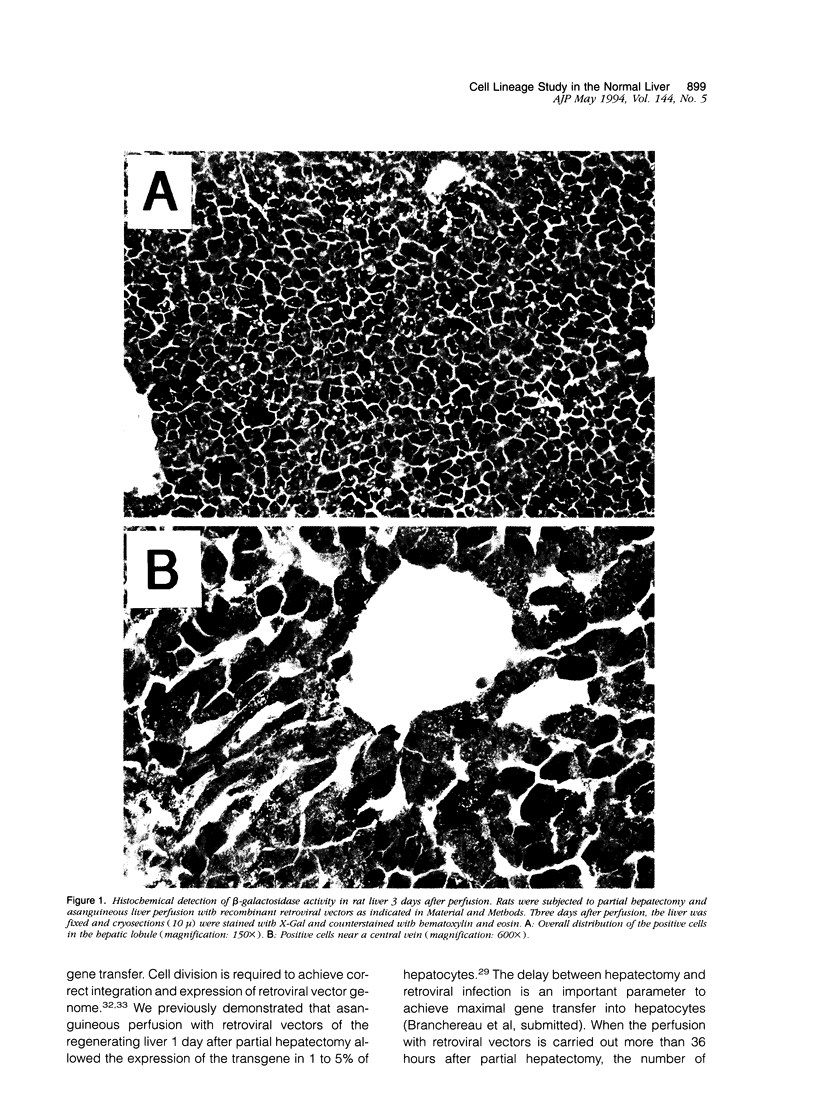
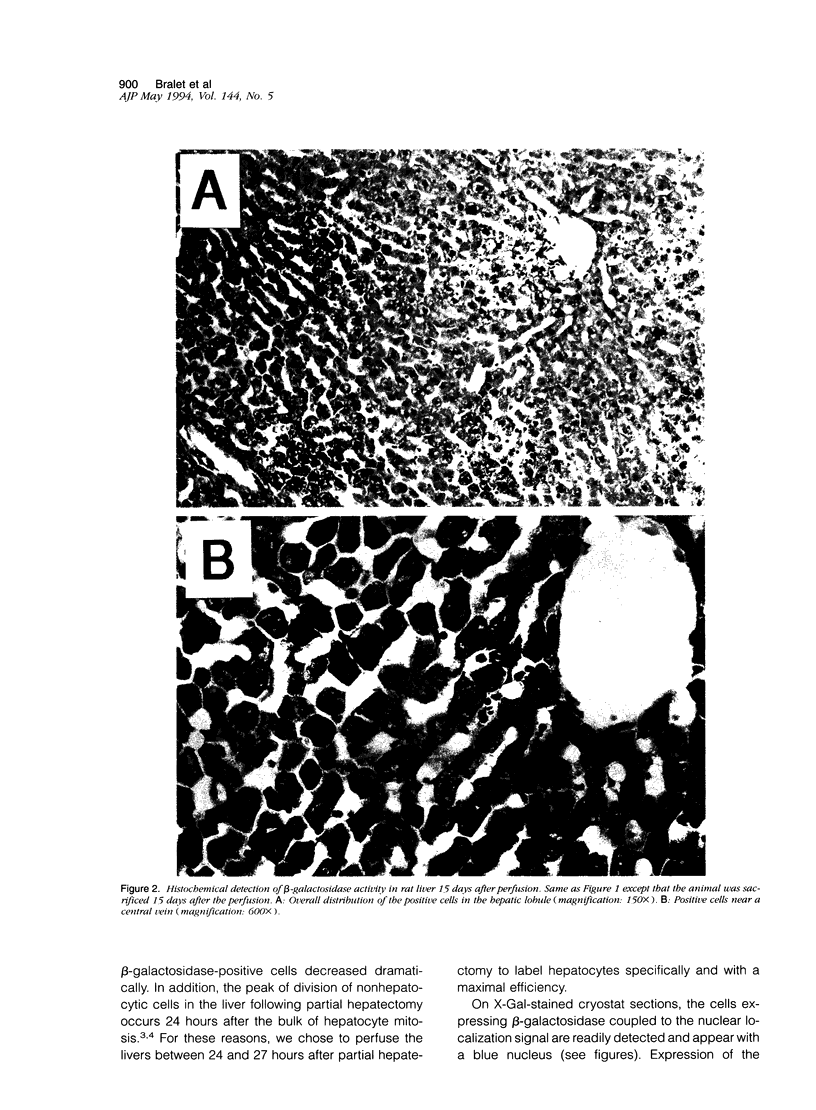


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber N., Zajicek G., Ariel I. The streaming liver. II. Hepatocyte life history. Liver. 1988 Apr;8(2):80–87. doi: 10.1111/j.1600-0676.1988.tb00972.x. [DOI] [PubMed] [Google Scholar]
- BRYANT B. J. Reutilization of leukocyte DNA by cells of regenerating liver. Exp Cell Res. 1962 Jun;27:70–79. doi: 10.1016/0014-4827(62)90044-7. [DOI] [PubMed] [Google Scholar]
- Becker F. F., Sell S. Early elevation of alpha 1-fetoprotein in N-2-fluorenylacetamide hepatocarcinogenesis. Cancer Res. 1974 Oct;34(10):2489–2494. [PubMed] [Google Scholar]
- Coleman W. B., Wennerberg A. E., Smith G. J., Grisham J. W. Regulation of the differentiation of diploid and some aneuploid rat liver epithelial (stemlike) cells by the hepatic microenvironment. Am J Pathol. 1993 May;142(5):1373–1382. [PMC free article] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Marsden E., Thorgeirsson S. S. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987 Nov;8(11):1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- FARBER E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956 Feb;16(2):142–148. [PubMed] [Google Scholar]
- Fabrikant J. I. The kinetics of cellular proliferation in regenerating liver. J Cell Biol. 1968 Mar;36(3):551–565. doi: 10.1083/jcb.36.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry N., Duplessis O., Houssin D., Danos O., Heard J. M. Retroviral-mediated gene transfer into hepatocytes in vivo. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8377–8381. doi: 10.1073/pnas.88.19.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRISHAM J. W. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-H3. Cancer Res. 1962 Aug;22:842–849. [PubMed] [Google Scholar]
- Gebhardt R. Altered acinar distribution of glutamine synthetase and different growth response of cultured enzyme-positive and -negative hepatocytes after partial hepatectomy. Cancer Res. 1990 Jul 15;50(14):4407–4410. [PubMed] [Google Scholar]
- Gebhardt R., Cruise J., Houck K. A., Luetteke N. C., Novotny A., Thaler F., Michalopoulos G. K. Differential effect of growth factors on growth stimulation and phenotypic stability of glutamine-synthetase-positive and -negative hepatocytes in primary culture. Differentiation. 1986;33(1):45–55. doi: 10.1111/j.1432-0436.1986.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Heiniger H. J., Friedrich G., Feinendegen L. E., Cantelmo F. Reutilization of 5- 125 I-iodo-2'-deoxyuridine and 3 H-thymidine in regenerating liver of mice. Proc Soc Exp Biol Med. 1971 Sep;137(4):1381–1384. doi: 10.3181/00379727-137-35793. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989 Jul;69(3):708–764. doi: 10.1152/physrev.1989.69.3.708. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kuo F. C., Darnell J. E., Jr Evidence that interaction of hepatocytes with the collecting (hepatic) veins triggers position-specific transcription of the glutamine synthetase and ornithine aminotransferase genes in the mouse liver. Mol Cell Biol. 1991 Dec;11(12):6050–6058. doi: 10.1128/mcb.11.12.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire J. M., Shiojiri N., Fausto N. Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am J Pathol. 1991 Sep;139(3):535–552. [PMC free article] [PubMed] [Google Scholar]
- Lemischka I. R. Retroviral lineage studies: some principals and applications. Curr Opin Genet Dev. 1993 Feb;3(1):115–118. doi: 10.1016/s0959-437x(05)80351-x. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R. What we have learned from retroviral marking of hematopoietic stem cells. Curr Top Microbiol Immunol. 1992;177:59–71. doi: 10.1007/978-3-642-76912-2_5. [DOI] [PubMed] [Google Scholar]
- MACDONALD R. A. "Lifespan" of liver cells. Autoradio-graphic study using tritiated thymidine in normal, cirrhotic, and partially hepatectomized rats. Arch Intern Med. 1961 Mar;107:335–343. doi: 10.1001/archinte.1961.03620030023003. [DOI] [PubMed] [Google Scholar]
- Marceau N. Cell lineages and differentiation programs in epidermal, urothelial and hepatic tissues and their neoplasms. Lab Invest. 1990 Jul;63(1):4–20. [PubMed] [Google Scholar]
- Miller D. G., Adam M. A., Miller A. D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990 Aug;10(8):4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan G. P., Fiering S., Nicolas J. F., Herzenberg L. A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-D-galactosidase activity after transduction of Escherichia coli lacZ. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff P. M., Ikeda T., Hixson D. C., Yam A. Characterizations of and interactions between bile ductule cells and hepatocytes in early stages of rat hepatocarcinogenesis induced by ethionine. Am J Pathol. 1991 Dec;139(6):1351–1368. [PMC free article] [PubMed] [Google Scholar]
- Petropoulos C. J., Yaswen P., Panzica M., Fausto N. Cell lineages in liver carcinogenesis: possible clues from studies of the distribution of alpha-fetoprotein RNA sequences in cell populations isolated from normal, regenerating, and preneoplastic rat livers. Cancer Res. 1985 Nov;45(11 Pt 2):5762–5768. [PubMed] [Google Scholar]
- RAPPAPORT A. M., BOROWY Z. J., LOUGHEED W. M., LOTTO W. N. Subdivision of hexagonal liver lobules into a structural and functional unit; role in hepatic physiology and pathology. Anat Rec. 1954 May;119(1):11–33. doi: 10.1002/ar.1091190103. [DOI] [PubMed] [Google Scholar]
- Rabes H. M., Wirsching R., Tuczek H. V., Iseler G. Analysis of cell cycle compartments of hepatocytes after partial hepatecomy. Cell Tissue Kinet. 1976 Nov;9(6):517–532. doi: 10.1111/j.1365-2184.1976.tb01301.x. [DOI] [PubMed] [Google Scholar]
- Roe T., Reynolds T. C., Yu G., Brown P. O. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993 May;12(5):2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S., Dunsford H. A. Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989 Jun;134(6):1347–1363. [PMC free article] [PubMed] [Google Scholar]
- Sell S. Is there a liver stem cell? Cancer Res. 1990 Jul 1;50(13):3811–3815. [PubMed] [Google Scholar]
- Sigal S. H., Brill S., Fiorino A. S., Reid L. M. The liver as a stem cell and lineage system. Am J Physiol. 1992 Aug;263(2 Pt 1):G139–G148. doi: 10.1152/ajpgi.1992.263.2.G139. [DOI] [PubMed] [Google Scholar]
- Thurman R. G., Kauffman F. C. Sublobular compartmentation of pharmacologic events (SCOPE): metabolic fluxes in periportal and pericentral regions of the liver lobule. Hepatology. 1985 Jan-Feb;5(1):144–151. doi: 10.1002/hep.1840050128. [DOI] [PubMed] [Google Scholar]
- Zajicek G., Oren R., Weinreb M., Jr The streaming liver. Liver. 1985 Dec;5(6):293–300. doi: 10.1111/j.1600-0676.1985.tb00252.x. [DOI] [PubMed] [Google Scholar]