Abstract
Interstitial inflammation and tubular injury accompany most types of glomerulonephritis and are likely to mediate progressive renal injury. We hypothesized that the interstitial monocyte/macrophage accumulation in nephritis involves osteopontin, a cell attachment glycoprotein that avidly binds macrophages in vitro and induces a macrophage-rich infiltrate on subcutaneous injection in mice (Singh et al, J Exp Med, 1990, 171: 1931). In this study, we demonstrate that osteopontin messenger RNA and protein levels are up-regulated in a proportion of proximal and distal tubules in three experimental models of glomerulonephritis. In all three models, the expression of osteopontin initially precedes histological evidence of tubular injury, but is correlated with subsequent sites of monocyte/macrophage accumulation and tubular damage. Osteopontin expression also correlates with the severity of the tubulointerstitial injury, being greatest in amino-nucleoside nephrosis. These data suggest that 1) osteopontin is up-regulated in tubules in glomerular disease; 2) osteopontin may be important for macrophage accumulation at specific sites in diseased tissue; and 3) osteopontin may therefore have a role in the pathogenesis of the tubulointerstitial injury that accompanies glomerulonephritis.
Full text
PDF




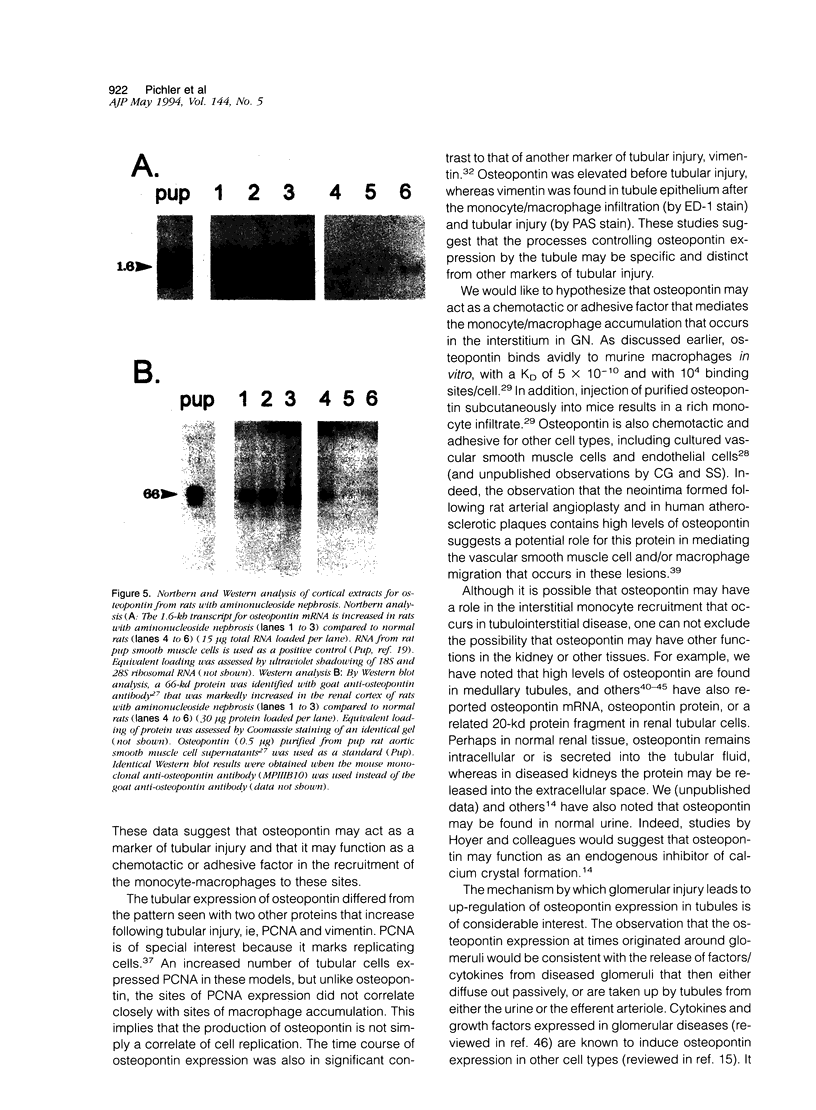




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud H. E. Growth factors in glomerulonephritis. Kidney Int. 1993 Jan;43(1):252–267. doi: 10.1038/ki.1993.39. [DOI] [PubMed] [Google Scholar]
- Alexopoulos E., Seron D., Hartley R. B., Cameron J. S. Lupus nephritis: correlation of interstitial cells with glomerular function. Kidney Int. 1990 Jan;37(1):100–109. doi: 10.1038/ki.1990.14. [DOI] [PubMed] [Google Scholar]
- Bohle A., Mackensen-Haen S., von Gise H. Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: a morphometric contribution. Am J Nephrol. 1987;7(6):421–433. doi: 10.1159/000167514. [DOI] [PubMed] [Google Scholar]
- Bohle A., Wehrmann M., Bogenschütz O., Batz C., Vogl W., Schmitt H., Müller C. A., Müller G. A. The long-term prognosis of the primary glomerulonephritides. A morphological and clinical analysis of 1747 cases. Pathol Res Pract. 1992 Oct;188(7):908–924. doi: 10.1016/s0344-0338(11)80252-9. [DOI] [PubMed] [Google Scholar]
- Brown L. F., Berse B., Van de Water L., Papadopoulos-Sergiou A., Perruzzi C. A., Manseau E. J., Dvorak H. F., Senger D. R. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell. 1992 Oct;3(10):1169–1180. doi: 10.1091/mbc.3.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. T. The nature and significance of osteopontin. Connect Tissue Res. 1989;23(2-3):123–136. doi: 10.3109/03008208909002412. [DOI] [PubMed] [Google Scholar]
- Craig A. M., Denhardt D. T. The murine gene encoding secreted phosphoprotein 1 (osteopontin): promoter structure, activity, and induction in vivo by estrogen and progesterone. Gene. 1991 Apr;100:163–171. doi: 10.1016/0378-1119(91)90362-f. [DOI] [PubMed] [Google Scholar]
- Craig A. M., Smith J. H., Denhardt D. T. Osteopontin, a transformation-associated cell adhesion phosphoprotein, is induced by 12-O-tetradecanoylphorbol 13-acetate in mouse epidermis. J Biol Chem. 1989 Jun 5;264(16):9682–9689. [PubMed] [Google Scholar]
- Diamond J. R., Anderson S. Irreversible tubulointerstitial damage associated with chronic aminonucleoside nephrosis. Amelioration by angiotensin I converting enzyme inhibition. Am J Pathol. 1990 Dec;137(6):1323–1332. [PMC free article] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Floege J., Johnson R. J., Gordon K., Iida H., Pritzl P., Yoshimura A., Campbell C., Alpers C. E., Couser W. G. Increased synthesis of extracellular matrix in mesangial proliferative nephritis. Kidney Int. 1991 Sep;40(3):477–488. doi: 10.1038/ki.1991.235. [DOI] [PubMed] [Google Scholar]
- Giachelli C. M., Bae N., Almeida M., Denhardt D. T., Alpers C. E., Schwartz S. M. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993 Oct;92(4):1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachelli C. M., Pichler R., Lombardi D., Denhardt D. T., Alpers C. E., Schwartz S. M., Johnson R. J. Osteopontin expression in angiotensin II-induced tubulointerstitial nephritis. Kidney Int. 1994 Feb;45(2):515–524. doi: 10.1038/ki.1994.67. [DOI] [PubMed] [Google Scholar]
- Gröne H. J., Weber K., Gröne E., Helmchen U., Osborn M. Coexpression of keratin and vimentin in damaged and regenerating tubular epithelia of the kidney. Am J Pathol. 1987 Oct;129(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Hooke D. H., Gee D. C., Atkins R. C. Leukocyte analysis using monoclonal antibodies in human glomerulonephritis. Kidney Int. 1987 Apr;31(4):964–972. doi: 10.1038/ki.1987.93. [DOI] [PubMed] [Google Scholar]
- Jin C. H., Miyaura C., Ishimi Y., Hong M. H., Sato T., Abe E., Suda T. Interleukin 1 regulates the expression of osteopontin mRNA by osteoblasts. Mol Cell Endocrinol. 1990 Dec 21;74(3):221–228. doi: 10.1016/0303-7207(90)90227-y. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Alpers C. E., Yoshimura A., Lombardi D., Pritzl P., Floege J., Schwartz S. M. Renal injury from angiotensin II-mediated hypertension. Hypertension. 1992 May;19(5):464–474. doi: 10.1161/01.hyp.19.5.464. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Iida H., Alpers C. E., Majesky M. W., Schwartz S. M., Pritzi P., Gordon K., Gown A. M. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991 Mar;87(3):847–858. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan H. Y., Nikolic-Paterson D. J., Zarama M., Vannice J. L., Atkins R. C. Suppression of experimental crescentic glomerulonephritis by the interleukin-1 receptor antagonist. Kidney Int. 1993 Feb;43(2):479–485. doi: 10.1038/ki.1993.70. [DOI] [PubMed] [Google Scholar]
- Lan H. Y., Paterson D. J., Atkins R. C. Initiation and evolution of interstitial leukocytic infiltration in experimental glomerulonephritis. Kidney Int. 1991 Sep;40(3):425–433. doi: 10.1038/ki.1991.229. [DOI] [PubMed] [Google Scholar]
- Liaw L., Almeida M., Hart C. E., Schwartz S. M., Giachelli C. M. Osteopontin promotes vascular cell adhesion and spreading and is chemotactic for smooth muscle cells in vitro. Circ Res. 1994 Feb;74(2):214–224. doi: 10.1161/01.res.74.2.214. [DOI] [PubMed] [Google Scholar]
- Lopez C. A., Hoyer J. R., Wilson P. D., Waterhouse P., Denhardt D. T. Heterogeneity of osteopontin expression among nephrons in mouse kidneys and enhanced expression in sclerotic glomeruli. Lab Invest. 1993 Sep;69(3):355–363. [PubMed] [Google Scholar]
- Mark M. P., Prince C. W., Gay S., Austin R. L., Butler W. T. 44-kDal bone phosphoprotein (osteopontin) antigenicity at ectopic sites in newborn rats: kidney and nervous tissues. Cell Tissue Res. 1988 Jan;251(1):23–30. doi: 10.1007/BF00215443. [DOI] [PubMed] [Google Scholar]
- McCluskey R. T., Bhan A. K. Cell-mediated mechanisms in renal diseases. Kidney Int Suppl. 1982 May;11:S6–12. [PubMed] [Google Scholar]
- Miyazaki Y., Setoguchi M., Yoshida S., Higuchi Y., Akizuki S., Yamamoto S. The mouse osteopontin gene. Expression in monocytic lineages and complete nucleotide sequence. J Biol Chem. 1990 Aug 25;265(24):14432–14438. [PubMed] [Google Scholar]
- Nath K. A. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992 Jul;20(1):1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- Nemir M., DeVouge M. W., Mukherjee B. B. Normal rat kidney cells secrete both phosphorylated and nonphosphorylated forms of osteopontin showing different physiological properties. J Biol Chem. 1989 Oct 25;264(30):18202–18208. [PubMed] [Google Scholar]
- Nomura S., Wills A. J., Edwards D. R., Heath J. K., Hogan B. L. Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol. 1988 Feb;106(2):441–450. doi: 10.1083/jcb.106.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata K., Kurki P., Celis J. E., Nakamura R. M., Tan E. M. Monoclonal antibodies to a nuclear protein (PCNA/cyclin) associated with DNA replication. Exp Cell Res. 1987 Feb;168(2):475–486. doi: 10.1016/0014-4827(87)90020-6. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Park M. H., D'Agati V., Appel G. B., Pirani C. L. Tubulointerstitial disease in lupus nephritis: relationship to immune deposits, interstitial inflammation, glomerular changes, renal function, and prognosis. Nephron. 1986;44(4):309–319. doi: 10.1159/000184012. [DOI] [PubMed] [Google Scholar]
- Patarca R., Freeman G. J., Singh R. P., Wei F. Y., Durfee T., Blattner F., Regnier D. C., Kozak C. A., Mock B. A., Morse H. C., 3rd Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. J Exp Med. 1989 Jul 1;170(1):145–161. doi: 10.1084/jem.170.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince C. W., Oosawa T., Butler W. T., Tomana M., Bhown A. S., Bhown M., Schrohenloher R. E. Isolation, characterization, and biosynthesis of a phosphorylated glycoprotein from rat bone. J Biol Chem. 1987 Feb 25;262(6):2900–2907. [PubMed] [Google Scholar]
- Risdon R. A., Sloper J. C., De Wardener H. E. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968 Aug 17;2(7564):363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- Ross F. P., Chappel J., Alvarez J. I., Sander D., Butler W. T., Farach-Carson M. C., Mintz K. A., Robey P. G., Teitelbaum S. L., Cheresh D. A. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J Biol Chem. 1993 May 5;268(13):9901–9907. [PubMed] [Google Scholar]
- Saito T., Atkins R. C. Contribution of mononuclear leucocytes to the progression of experimental focal glomerular sclerosis. Kidney Int. 1990 Apr;37(4):1076–1083. doi: 10.1038/ki.1990.88. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Gracey C. F., Papadopoulos A., Tenen D. G. Secreted phosphoproteins associated with neoplastic transformation: close homology with plasma proteins cleaved during blood coagulation. Cancer Res. 1988 Oct 15;48(20):5770–5774. [PubMed] [Google Scholar]
- Shiraga H., Min W., VanDusen W. J., Clayman M. D., Miner D., Terrell C. H., Sherbotie J. R., Foreman J. W., Przysiecki C., Neilson E. G. Inhibition of calcium oxalate crystal growth in vitro by uropontin: another member of the aspartic acid-rich protein superfamily. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):426–430. doi: 10.1073/pnas.89.1.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., DeVouge M. W., Mukherjee B. B. Physiological properties and differential glycosylation of phosphorylated and nonphosphorylated forms of osteopontin secreted by normal rat kidney cells. J Biol Chem. 1990 Oct 25;265(30):18696–18701. [PubMed] [Google Scholar]
- Singh R. P., Patarca R., Schwartz J., Singh P., Cantor H. Definition of a specific interaction between the early T lymphocyte activation 1 (Eta-1) protein and murine macrophages in vitro and its effect upon macrophages in vivo. J Exp Med. 1990 Jun 1;171(6):1931–1942. doi: 10.1084/jem.171.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerman M. J., Fisher L. W., Foster R. A., Sauk J. J. Human bone sialoprotein I and II enhance fibroblast attachment in vitro. Calcif Tissue Int. 1988 Jul;43(1):50–53. doi: 10.1007/BF02555169. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Ullrich O., Mann K., Haase W., Koch-Brandt C. Biosynthesis and secretion of an osteopontin-related 20-kDa polypeptide in the Madin-Darby canine kidney cell line. J Biol Chem. 1991 Feb 25;266(6):3518–3525. [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Schwartz S. M., Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee J., Kuncio G. S., Neilson E. G. Tubulointerstitial injury following glomerulonephritis. Semin Nephrol. 1991 May;11(3):361–366. [PubMed] [Google Scholar]
- Yoon K., Buenaga R., Rodan G. A. Tissue specificity and developmental expression of rat osteopontin. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1129–1136. doi: 10.1016/s0006-291x(87)80250-4. [DOI] [PubMed] [Google Scholar]
- Young M. F., Kerr J. M., Termine J. D., Wewer U. M., Wang M. G., McBride O. W., Fisher L. W. cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN). Genomics. 1990 Aug;7(4):491–502. doi: 10.1016/0888-7543(90)90191-v. [DOI] [PubMed] [Google Scholar]