Abstract
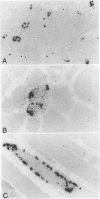
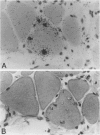
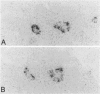
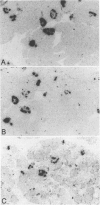
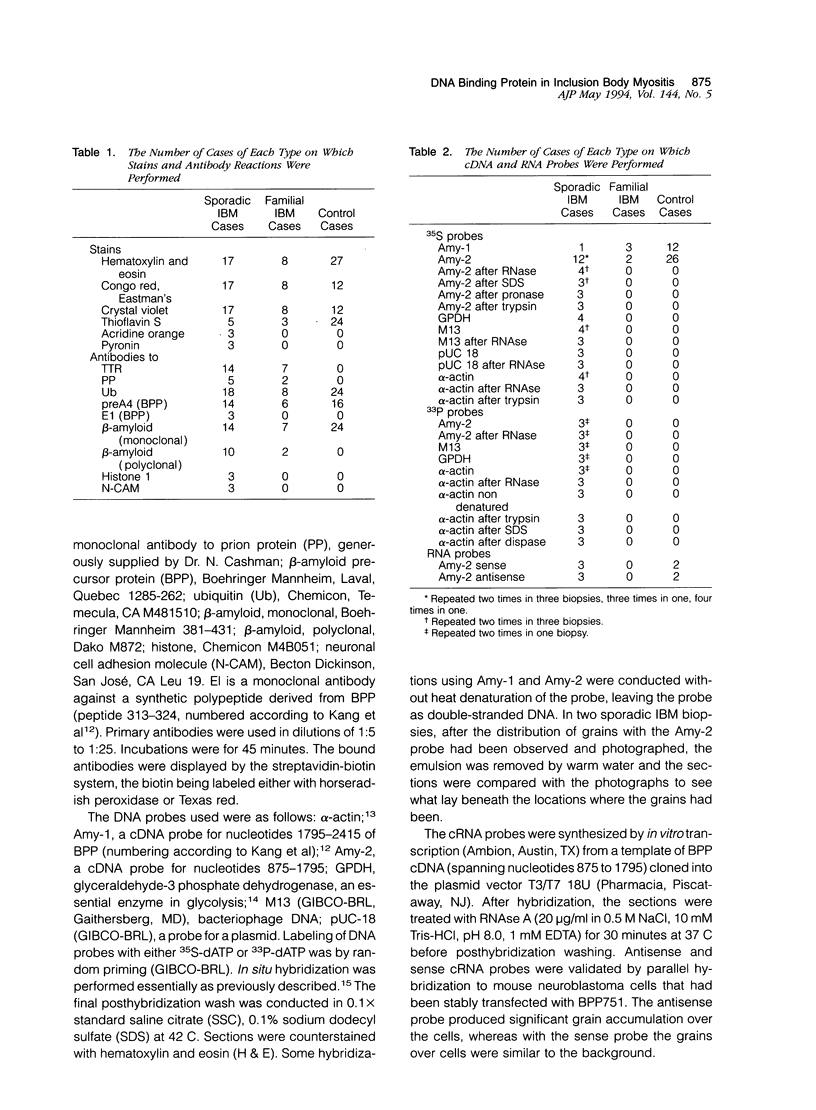
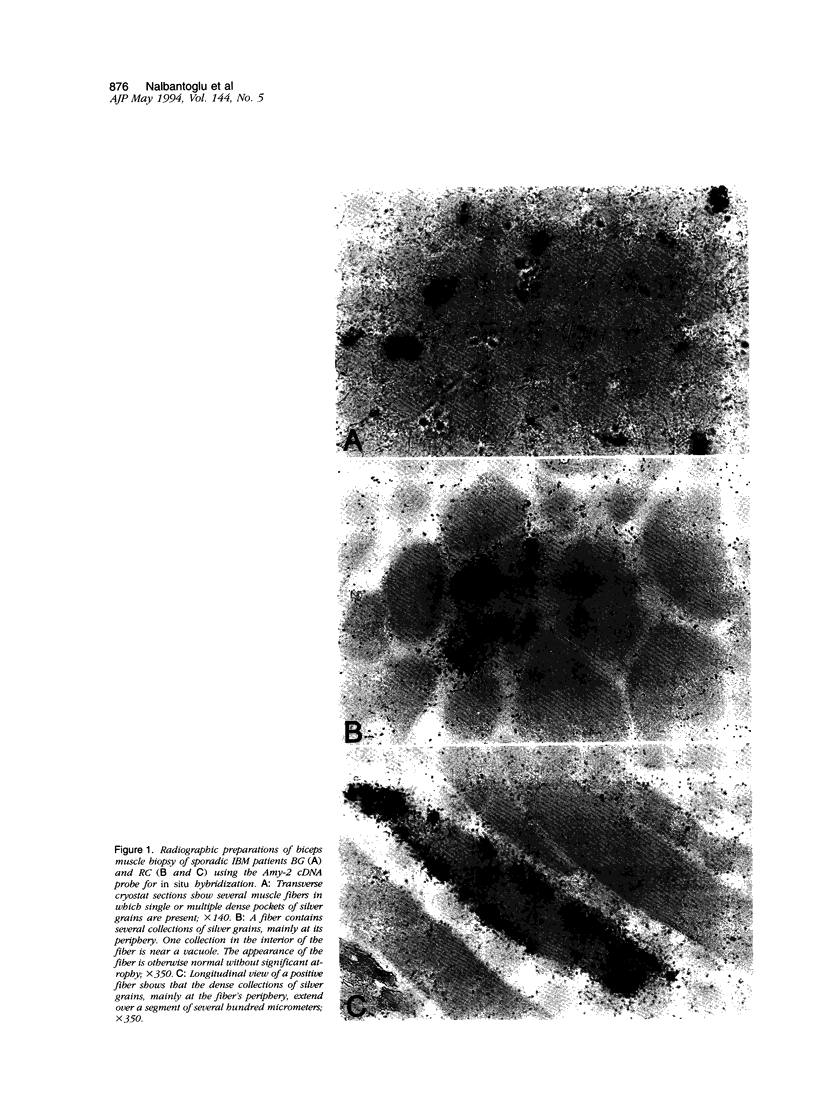
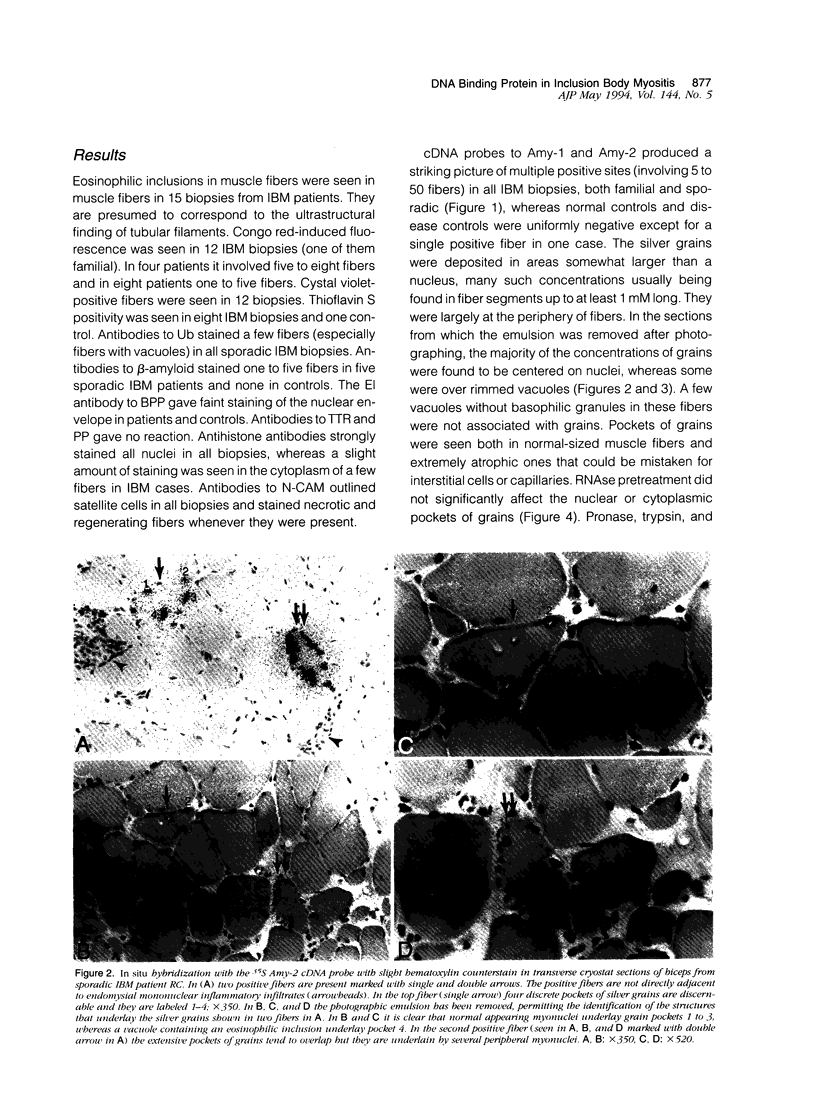
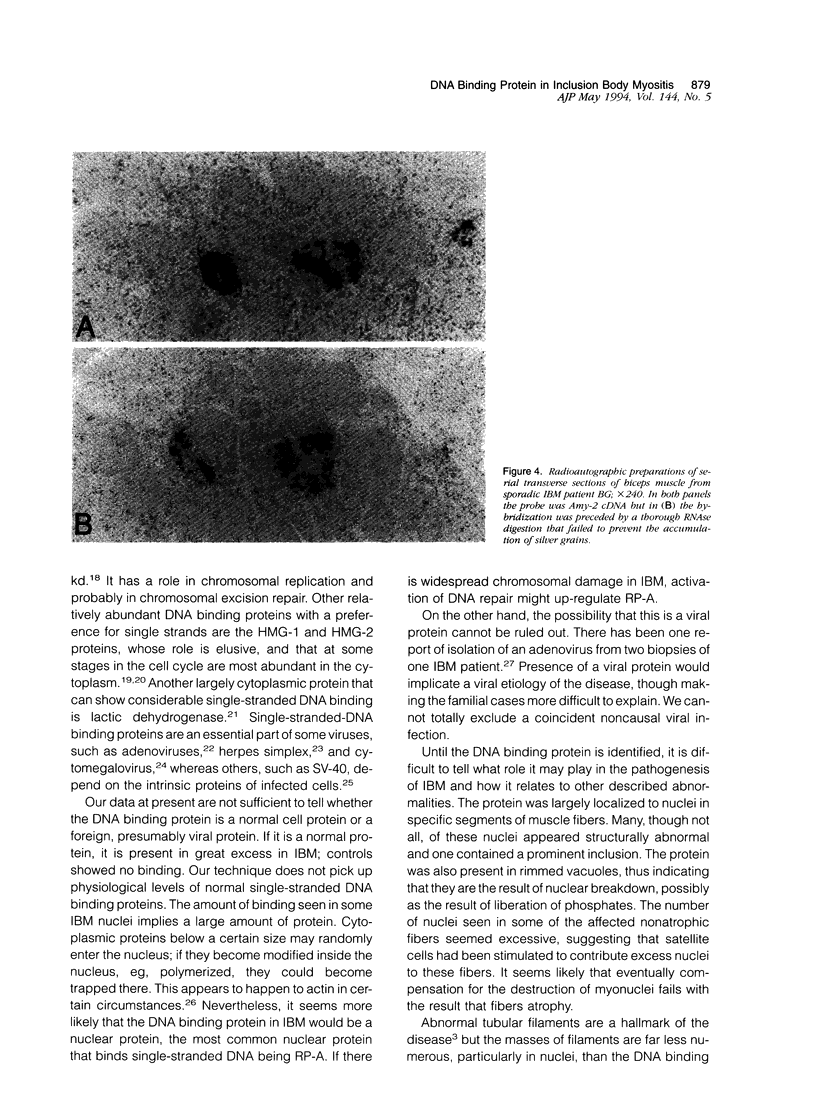
In muscle biopsies from patients with inclusion body myositis (IBM), multiple sites were found in many muscle fibers that bound single-stranded but not double-stranded DNA without sequence specificity, as exemplified by several different cDNA probes. This activity was attributable to a protein, because it was abolished by proteases but not by RNAse. Most of the sites of binding were myonuclei, whereas some were rimmed vacuoles, which probably result from nuclear breakdown. No comparable binding was seen in 27 control biopsies. A number of human and viral single-stranded DNA binding proteins exist but our data does not identify the protein responsible for DNA binding in IBM. Our findings reinforce the supposition that nuclear damage plays a basic role in the pathogenesis of IBM.
Full text
PDF

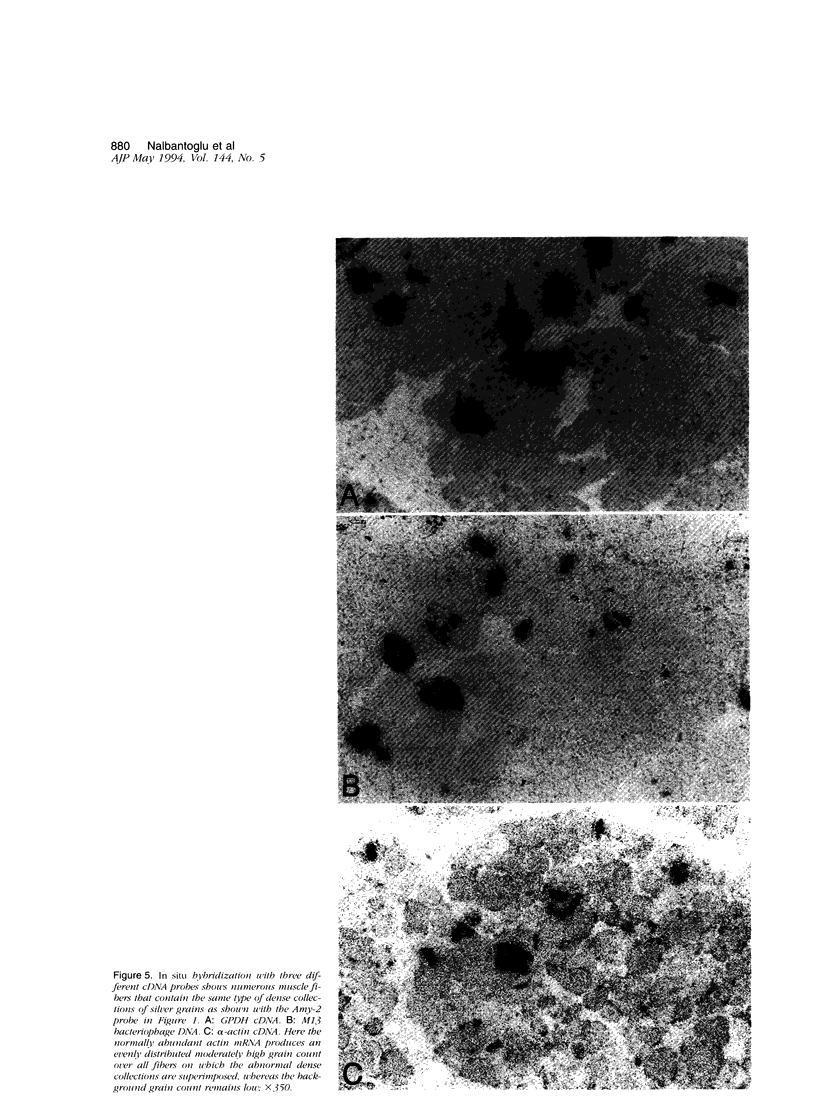


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders D. G. Nucleotide sequence of a cytomegalovirus single-stranded DNA-binding protein gene: comparison with alpha- and gammaherpesvirus counterparts reveals conserved segments. J Gen Virol. 1990 Oct;71(Pt 10):2451–2456. doi: 10.1099/0022-1317-71-10-2451. [DOI] [PubMed] [Google Scholar]
- Askanas V., Engel W. K., Alvarez R. B. Light and electron microscopic localization of beta-amyloid protein in muscle biopsies of patients with inclusion-body myositis. Am J Pathol. 1992 Jul;141(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Askanas V., Engel W. K., Alvarez R. B. Strong immunoreactivity of beta-amyloid precursor protein, including the beta-amyloid protein sequence, at human neuromuscular junctions. Neurosci Lett. 1992 Aug 31;143(1-2):96–100. doi: 10.1016/0304-3940(92)90241-x. [DOI] [PubMed] [Google Scholar]
- Askanas V., Serdaroglu P., Engel W. K., Alvarez R. B. Immunolocalization of ubiquitin in muscle biopsies of patients with inclusion body myositis and oculopharyngeal muscular dystrophy. Neurosci Lett. 1991 Sep 2;130(1):73–76. doi: 10.1016/0304-3940(91)90230-q. [DOI] [PubMed] [Google Scholar]
- Bilak M., Askanas V., Engel W. K. Strong immunoreactivity of alpha 1-antichymotrypsin co-localizes with beta-amyloid protein and ubiquitin in vacuolated muscle fibers of inclusion-body myositis. Acta Neuropathol. 1993;85(4):378–382. doi: 10.1007/BF00334447. [DOI] [PubMed] [Google Scholar]
- Bustin M., Lehn D. A., Landsman D. Structural features of the HMG chromosomal proteins and their genes. Biochim Biophys Acta. 1990 Jul 30;1049(3):231–243. doi: 10.1016/0167-4781(90)90092-g. [DOI] [PubMed] [Google Scholar]
- Carpenter S., Karpati G., Heller I., Eisen A. Inclusion body myositis: a distinct variety of idiopathic inflammatory myopathy. Neurology. 1978 Jan;28(1):8–17. doi: 10.1212/wnl.28.1.8. [DOI] [PubMed] [Google Scholar]
- Carpenter S., Karpati G. The major inflammatory myopathies of unknown cause. Pathol Annu. 1981;16(Pt 2):205–237. [PubMed] [Google Scholar]
- Carpenter S., Karpati G., Wolfe L. Virus-like filaments and phospholipid accumulation in skeletal muscle. Study of a histochemically distinct chronic myopathy. Neurology. 1970 Sep;20(9):889–903. doi: 10.1212/wnl.20.9.989. [DOI] [PubMed] [Google Scholar]
- Chou S. M. Myxovirus-like structures and accompanying nuclear changes in chronic polymyositis. Arch Pathol. 1968 Dec;86(6):649–658. [PubMed] [Google Scholar]
- Cole A. J., Kuzniecky R., Karpati G., Carpenter S., Andermann E., Andermann F. Familial myopathy with changes resembling inclusion body myositis and periventricular leucoencephalopathy. A new syndrome. Brain. 1988 Oct;111(Pt 5):1025–1037. doi: 10.1093/brain/111.5.1025. [DOI] [PubMed] [Google Scholar]
- Danon J. M., Karpati G., Carpenter S. Subacute skeletal myopathy induced by 2,4-dichlorophenoxyacetate in rats and guinea pigs. Muscle Nerve. 1978 Mar-Apr;1(2):89–102. doi: 10.1002/mus.880010202. [DOI] [PubMed] [Google Scholar]
- Eagle P. A., Klessig D. F. A zinc-binding motif located between amino acids 273 and 286 in the adenovirus DNA-binding protein is necessary for ssDNA binding. Virology. 1992 Apr;187(2):777–787. doi: 10.1016/0042-6822(92)90479-9. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerusalem F., Baumgartner G., Wyler R. Virus-ähnliche Einschlüsse bei chronischen neuro-muskulären Prozessen. Elektronenmikroskopische Biopsiebefunde von 2 Fällen. Arch Psychiatr Nervenkr (1970) 1972;215(2):148–166. doi: 10.1007/BF00342531. [DOI] [PubMed] [Google Scholar]
- Kaiserman H. B., Odenwald W. F., Stowers D. J., Poll E. H., Benbow R. M. A major single-stranded DNA binding protein from ovaries of the frog, Xenopus laevis, is lactate dehydrogenase. Biochim Biophys Acta. 1989 Jun 1;1008(1):23–30. doi: 10.1016/0167-4781(89)90165-6. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Koppe R. I., Hallauer P. L., Karpati G., Hastings K. E. cDNA clone and expression analysis of rodent fast and slow skeletal muscle troponin I mRNAs. J Biol Chem. 1989 Aug 25;264(24):14327–14333. [PubMed] [Google Scholar]
- Lotz B. P., Engel A. G., Nishino H., Stevens J. C., Litchy W. J. Inclusion body myositis. Observations in 40 patients. Brain. 1989 Jun;112(Pt 3):727–747. doi: 10.1093/brain/112.3.727. [DOI] [PubMed] [Google Scholar]
- Massa R., Weller B., Karpati G., Shoubridge E., Carpenter S. Familial inclusion body myositis among Kurdish-Iranian Jews. Arch Neurol. 1991 May;48(5):519–522. doi: 10.1001/archneur.1991.00530170083024. [DOI] [PubMed] [Google Scholar]
- Melendy T., Stillman B. An interaction between replication protein A and SV40 T antigen appears essential for primosome assembly during SV40 DNA replication. J Biol Chem. 1993 Feb 15;268(5):3389–3395. [PubMed] [Google Scholar]
- Mendell J. R., Sahenk Z., Gales T., Paul L. Amyloid filaments in inclusion body myositis. Novel findings provide insight into nature of filaments. Arch Neurol. 1991 Dec;48(12):1229–1234. doi: 10.1001/archneur.1991.00530240033013. [DOI] [PubMed] [Google Scholar]
- Mikol J., Felten-Papaiconomou A., Ferchal F., Perol Y., Gautier B., Haguenau M., Pepin B. Inclusion-body myositis: clinicopathological studies and isolation of an adenovirus type 2 from muscle biopsy specimen. Ann Neurol. 1982 Jun;11(6):576–581. doi: 10.1002/ana.410110605. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Olson J. W. Surface lysine and tyrosine residues are required for interaction of the major herpes simplex virus type 1 DNA-binding protein with single-stranded DNA. J Virol. 1992 Nov;66(11):6273–6279. doi: 10.1128/jvi.66.11.6273-6279.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkozi E., Askanas V., Johnson S. A., Engel W. K., Alvarez R. B. beta-Amyloid precursor protein mRNA is increased in inclusion-body myositis muscle. Neuroreport. 1993 Jun;4(6):815–818. doi: 10.1097/00001756-199306000-00055. [DOI] [PubMed] [Google Scholar]
- Sato T., Walker D. L., Peters H. A., Resse H. H., Chou S. M. Chronic polymyositis and myxovirus-like inclusions. Electron microscopic and viral studies. Arch Neurol. 1971 May;24(5):409–418. doi: 10.1001/archneur.1971.00480350043004. [DOI] [PubMed] [Google Scholar]
- Seroussi E., Lavi S. Replication protein A is the major single-stranded DNA binding protein detected in mammalian cell extracts by gel retardation assays and UV cross-linking of long and short single-stranded DNA molecules. J Biol Chem. 1993 Apr 5;268(10):7147–7154. [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir H. M., Kraulis P. J., Hill C. S., Raine A. R., Laue E. D., Thomas J. O. Structure of the HMG box motif in the B-domain of HMG1. EMBO J. 1993 Apr;12(4):1311–1319. doi: 10.1002/j.1460-2075.1993.tb05776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis E. J., Samaha F. J. Inclusion body myositis. Lab Invest. 1971 Sep;25(3):240–248. [PubMed] [Google Scholar]
- Zimmermann K., Herget T., Salbaum J. M., Schubert W., Hilbich C., Cramer M., Masters C. L., Multhaup G., Kang J., Lemaire H. G. Localization of the putative precursor of Alzheimer's disease-specific amyloid at nuclear envelopes of adult human muscle. EMBO J. 1988 Feb;7(2):367–372. doi: 10.1002/j.1460-2075.1988.tb02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]