Abstract
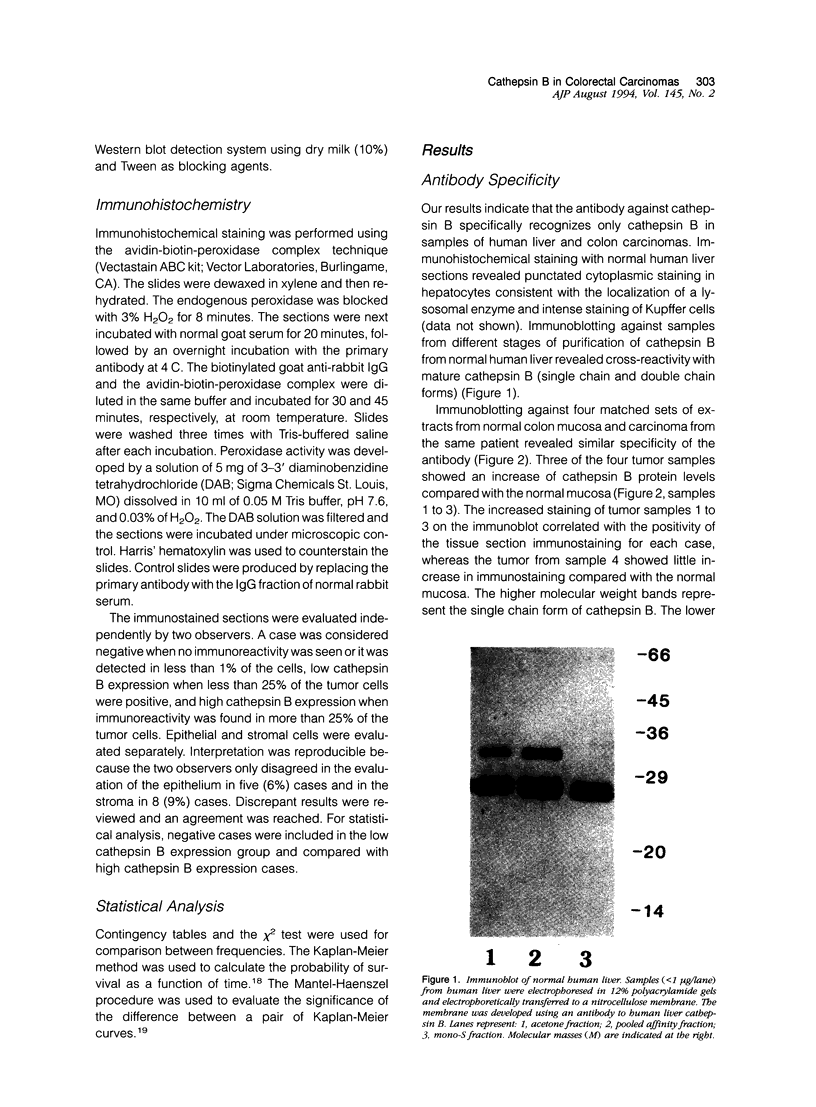
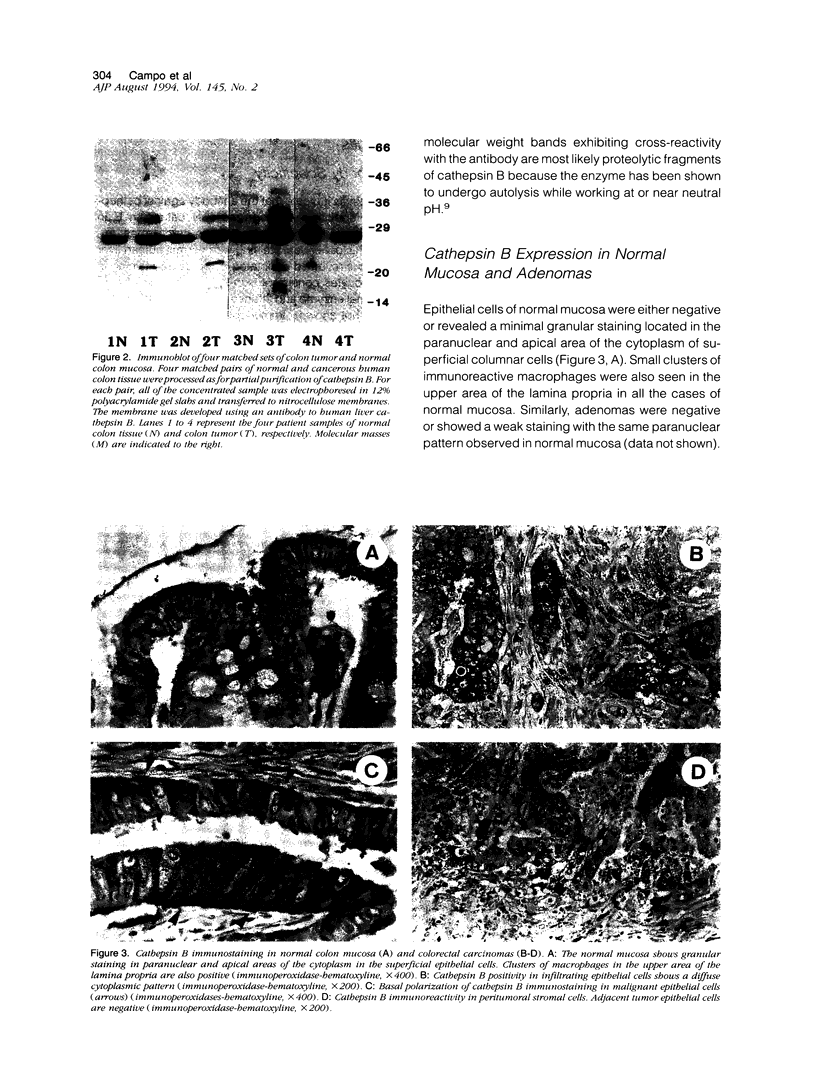
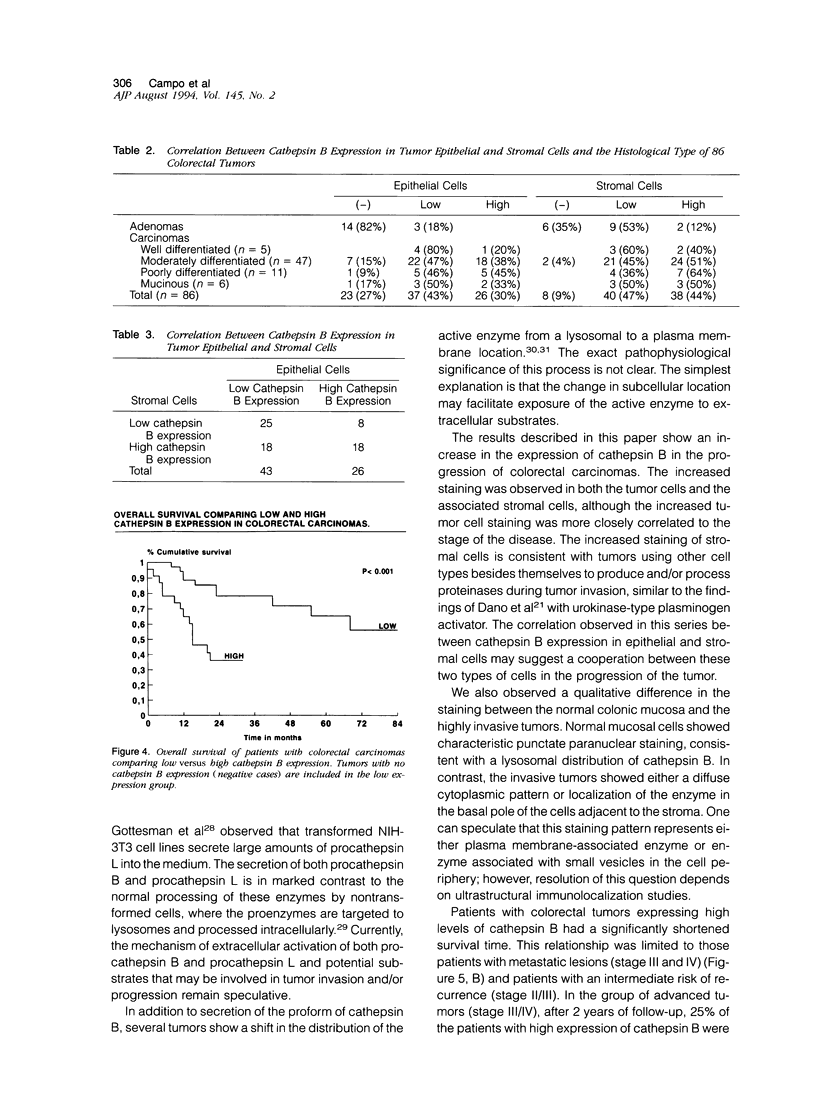
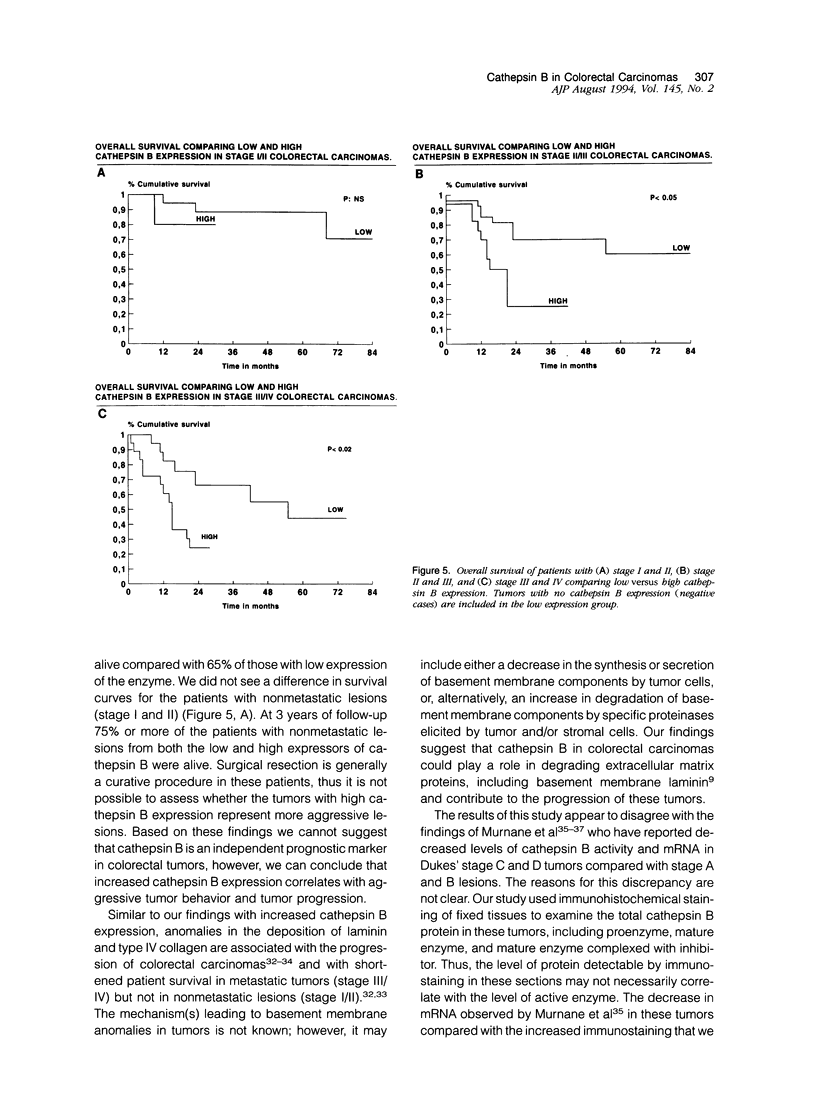
Cathepsin B is a lysosomal cysteine proteinase that has the ability to degrade several extracellular matrix components at both neutral and acidic pH and has been implicated in the progression of several human and rodent tumors. We have studied the expression of cathepsin B in human colorectal tissues using a monospecific polyclonal rabbit antibody raised against human liver cathepsin B. In immunoblots of normal and neoplastic colorectal tissues this antibody specifically recognized only cathepsin B. We studied 101 cases of formalin-fixed, paraffin-embedded tissue (15 normal mucosa, 17 adenomas, and 69 carcinomas). Epithelial cells of normal mucosa and adenomas were either negative or showed a weak granular reactivity located in the paranuclear and apical cytoplasm of superficial cells. Small clusters of histiocytes were also positive in the region of the superficial area of the lamina propria. In carcinomas, increased expression of cathepsin B correlated with advanced stage of the disease. Increased immunoreactivity of cathepsin B in malignant cells was associated with either a diffuse cytoplasmic staining or was polarized to the basal pole of the cells. This is in contrast to the punctate paranuclear staining pattern observed in normal colonic mucosal cells. In tumor stromal cells, increased expression of the enzyme correlated with neoplastic progression. Expression of high levels of cathepsin B in the tumor epithelial cells was associated with a significantly shorter survival of the patients. In conclusion, our results indicate that cathepsin B expression is up-regulated in human colorectal carcinomas compared with normal mucosa and adenomas and correlates with tumor progression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baici A., Knöpfel M., Keist R. Tumor-host interactions in the rabbit V2 carcinoma: stimulation of cathepsin B in host fibroblasts by a tumor-derived cytokine. Invasion Metastasis. 1988;8(3):143–158. [PubMed] [Google Scholar]
- Blasi F., Verde P. Urokinase-dependent cell surface proteolysis and cancer. Semin Cancer Biol. 1990 Apr;1(2):117–126. [PubMed] [Google Scholar]
- Buck M. R., Karustis D. G., Day N. A., Honn K. V., Sloane B. F. Degradation of extracellular-matrix proteins by human cathepsin B from normal and tumour tissues. Biochem J. 1992 Feb 15;282(Pt 1):273–278. doi: 10.1042/bj2820273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson A. H. Biosynthesis of lysosomal endopeptidases. J Cell Biochem. 1989 May;40(1):31–41. doi: 10.1002/jcb.240400104. [DOI] [PubMed] [Google Scholar]
- Forster S. J., Talbot I. C., Clayton D. G., Critchley D. R. Tumour basement membrane laminin in adenocarcinoma of rectum: an immunohistochemical study of biological and clinical significance. Int J Cancer. 1986 Jun 15;37(6):813–817. doi: 10.1002/ijc.2910370603. [DOI] [PubMed] [Google Scholar]
- Gray S. T., Wilkins R. J., Yun K. Interstitial collagenase gene expression in oral squamous cell carcinoma. Am J Pathol. 1992 Aug;141(2):301–306. [PMC free article] [PubMed] [Google Scholar]
- Havenith M. G., Arends J. W., Simon R., Volovics A., Wiggers T., Bosman F. T. Type IV collagen immunoreactivity in colorectal cancer. Prognostic value of basement membrane deposition. Cancer. 1988 Nov 15;62(10):2207–2211. doi: 10.1002/1097-0142(19881115)62:10<2207::aid-cncr2820621023>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Hewitt R. E., Powe D. G., Griffin N. R., Turner D. R. Relationships between epithelial basement membrane staining patterns in primary colorectal carcinomas and the extent of tumour spread. Int J Cancer. 1991 Jul 30;48(6):855–860. doi: 10.1002/ijc.2910480611. [DOI] [PubMed] [Google Scholar]
- Kane S. E., Gottesman M. M. The role of cathepsin L in malignant transformation. Semin Cancer Biol. 1990 Apr;1(2):127–136. [PubMed] [Google Scholar]
- Keppler D., Fondanèche M. C., Dalet-Fumeron V., Pagano M., Burtin P. Immunohistochemical and biochemical study of a cathepsin B-like proteinase in human colonic cancers. Cancer Res. 1988 Dec 1;48(23):6855–6862. [PubMed] [Google Scholar]
- Levy A. T., Cioce V., Sobel M. E., Garbisa S., Grigioni W. F., Liotta L. A., Stetler-Stevenson W. G. Increased expression of the Mr 72,000 type IV collagenase in human colonic adenocarcinoma. Cancer Res. 1991 Jan 1;51(1):439–444. [PubMed] [Google Scholar]
- Liotta L. A., Stetler-Stevenson W. G. Metalloproteinases and cancer invasion. Semin Cancer Biol. 1990 Apr;1(2):99–106. [PubMed] [Google Scholar]
- Maciewicz R. A., Wardale R. J., Etherington D. J., Paraskeva C. Immunodetection of cathepsins B and L present in and secreted from human pre-malignant and malignant colorectal tumour cell lines. Int J Cancer. 1989 Mar 15;43(3):478–486. doi: 10.1002/ijc.2910430323. [DOI] [PubMed] [Google Scholar]
- Maciewicz R. A., Wotton S. F., Etherington D. J., Duance V. C. Susceptibility of the cartilage collagens types II, IX and XI to degradation by the cysteine proteinases, cathepsins B and L. FEBS Lett. 1990 Aug 20;269(1):189–193. doi: 10.1016/0014-5793(90)81151-d. [DOI] [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- Mason R. W., Gal S., Gottesman M. M. The identification of the major excreted protein (MEP) from a transformed mouse fibroblast cell line as a catalytically active precursor form of cathepsin L. Biochem J. 1987 Dec 1;248(2):449–454. doi: 10.1042/bj2480449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Bowden G. T. Stromelysin/transin and tumor progression. Semin Cancer Biol. 1990 Apr;1(2):107–115. [PubMed] [Google Scholar]
- Moin K., Day N. A., Sameni M., Hasnain S., Hirama T., Sloane B. F. Human tumour cathepsin B. Comparison with normal liver cathepsin B. Biochem J. 1992 Jul 15;285(Pt 2):427–434. doi: 10.1042/bj2850427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort J. S., Leduc M., Recklies A. D. A latent thiol proteinase from ascitic fluid of patients with neoplasia. Biochim Biophys Acta. 1981 Dec 15;662(2):173–180. doi: 10.1016/0005-2744(81)90027-9. [DOI] [PubMed] [Google Scholar]
- Murnane M. J., Sheahan K., Ozdemirli M., Shuja S. Stage-specific increases in cathepsin B messenger RNA content in human colorectal carcinoma. Cancer Res. 1991 Feb 15;51(4):1137–1142. [PubMed] [Google Scholar]
- Ossowski L., Clunie G., Masucci M. T., Blasi F. In vivo paracrine interaction between urokinase and its receptor: effect on tumor cell invasion. J Cell Biol. 1991 Nov;115(4):1107–1112. doi: 10.1083/jcb.115.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsom R., Pignatelli M., Stetler-Stevenson W. G., Liotta L. A., Wright P. A., Jeffery R. E., Longcroft J. M., Rogers L., Stamp G. W. Stromal expression of 72 kda type IV collagenase (MMP-2) and TIMP-2 mRNAs in colorectal neoplasia. Am J Pathol. 1992 Aug;141(2):389–396. [PMC free article] [PubMed] [Google Scholar]
- Pyke C., Kristensen P., Ralfkiaer E., Grøndahl-Hansen J., Eriksen J., Blasi F., Danø K. Urokinase-type plasminogen activator is expressed in stromal cells and its receptor in cancer cells at invasive foci in human colon adenocarcinomas. Am J Pathol. 1991 May;138(5):1059–1067. [PMC free article] [PubMed] [Google Scholar]
- Recklies A. D., Poole A. R., Mort J. S. A cysteine proteinase secreted from human breast tumours is immunologically related to cathepsin B. Biochem J. 1982 Dec 1;207(3):633–636. doi: 10.1042/bj2070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recklies A. D., Tiltman K. J., Stoker T. A., Poole A. R. Secretion of proteinases from malignant and nonmalignant human breast tissue. Cancer Res. 1980 Mar;40(3):550–556. [PubMed] [Google Scholar]
- Rochefort H. Biological and clinical significance of cathepsin D in breast cancer. Semin Cancer Biol. 1990 Apr;1(2):153–160. [PubMed] [Google Scholar]
- Rozhin J., Robinson D., Stevens M. A., Lah T. T., Honn K. V., Ryan R. E., Sloane B. F. Properties of a plasma membrane-associated cathepsin B-like cysteine proteinase in metastatic B16 melanoma variants. Cancer Res. 1987 Dec 15;47(24 Pt 1):6620–6628. [PubMed] [Google Scholar]
- Sheahan K., Shuja S., Murnane M. J. Cysteine protease activities and tumor development in human colorectal carcinoma. Cancer Res. 1989 Jul 15;49(14):3809–3814. [PubMed] [Google Scholar]
- Shuja S., Sheahan K., Murnane M. J. Cysteine endopeptidase activity levels in normal human tissues, colorectal adenomas and carcinomas. Int J Cancer. 1991 Sep 30;49(3):341–346. doi: 10.1002/ijc.2910490305. [DOI] [PubMed] [Google Scholar]
- Sloane B. F. Cathepsin B and cystatins: evidence for a role in cancer progression. Semin Cancer Biol. 1990 Apr;1(2):137–152. [PubMed] [Google Scholar]
- Sloane B. F., Rozhin J., Johnson K., Taylor H., Crissman J. D., Honn K. V. Cathepsin B: association with plasma membrane in metastatic tumors. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2483–2487. doi: 10.1073/pnas.83.8.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]